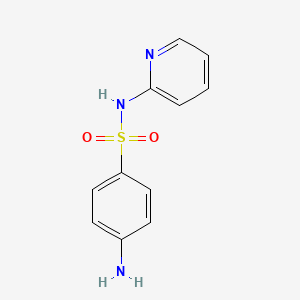
| InChI |
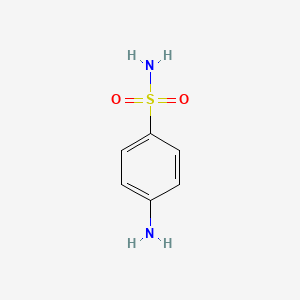
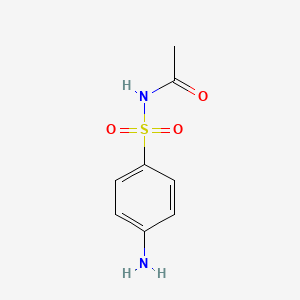
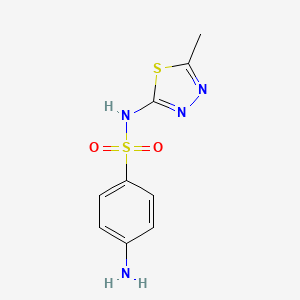
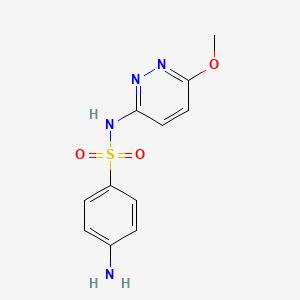
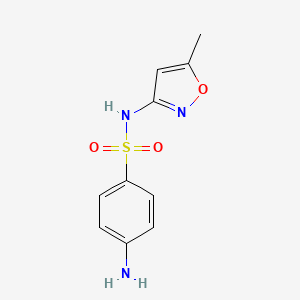
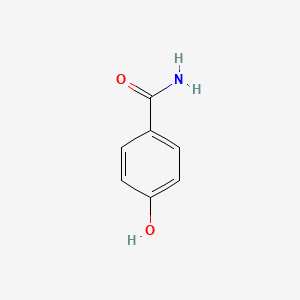
InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10)
|
| Synonyms |
sulfanilamide; 4-Aminobenzenesulfonamide; 63-74-1; Sulphanilamide; Sulfamine; p-Aminobenzenesulfonamide; Sulphonamide; Sulfonylamide; 4-Aminobenzene-1-Sulfonamide; p-Aminobenzenesulfamide; Prontylin; Prontosil Album; Bacteramid; Streptasol; Streptocid; p-Sulfamoylaniline; Sulfonamide P; Prontosil I; Benzenesulfonamide, 4-amino-; Estreptocida; Exoseptoplix; Streptoclase; p-Sulfamidoaniline; Sulfamidyl; Sulfanalone; Sulfanidyl; Sulfanil; Sulfocidine; Sulfana; 4-Sulfamoylaniline; Sulfanilimidic acid; p-Anilinesulfonamide; p-Aminophenylsulfonamide; Ambeside; Antistrept; Astreptine; Astrocid; Bactesid; Collomide; Colsulanyde; Copticide; Deseptyl; Ergaseptine; Erysipan; Gombardol; Lysococcine; Neococcyl; Orgaseptine; Prontalbin; Proseptal; Proseptine; Proseptol; Pysococcine; Septanilam; Septinal; Septolix; Septoplex; Septoplix; Strepamide; Strepsan; Streptagol; Streptamid; Streptamin; Streptocide; Streptocom; Strepton; Streptopan; Streptosil; Streptozol; Streptozone; Streptrocide; Sulfocidin; Therapol; Albexan; Albosal; Dipron; Gerison; Infepan; Sanamid; Stramid; Tolder; Lusil; Prontosil White; Pronzin Album; Septamide Album; Stopton Album; Streptocid album; 4-Aminophenylsulfonamide; Rubiazol A; White streptocide; PABS; Streptocide White; p-Aminobenzenesulfonylamide; Aniline-p-sulfonic amide; Fourneau 1162; Sulfanilamide Vaginal Cream; Benzenesulfonamide, p-amino-; 4-azanylbenzenesulfonamide; 4-Amino-benzenesulfonamide; MFCD00007939; p-Aminobenzensulfonamide; Sulphanilamidum; 1162 F; 4-(Aminosulfonyl)aniline; para-aminobenzenesulfonamide; A-349; F 1162; CHEBI:45373; 4-aminobenzene sulfonic acid amide; NSC-7618; CHEMBL21; p-amino benzene sulfonamide; (4-(Aminosulfonyl)phenyl)amine; AVC; Streptocidum; NSC7618; Sulfanilamide Melting Point Standard; Sulfanilamide (INN); 21240MF57M; CAS-63-74-1; NCGC00016285-02; NCGC00016285-05; Sulfanilamida; Sulfanilamidum; 4-Aminobenzenesulphonamide (Sulphanilamide); Sulfanilamide, >=99%; DSSTox_CID_3622; SULFANILAMIDE [INN]; DSSTox_RID_77115; DSSTox_GSID_23622; Streptocide (VAN); Solfanilamide [DCIT]; Caswell No. 809A; Solfanilamide; HSDB 223; Sulfanilamidum [INN-Latin]; Sulfanilamida [INN-Spanish]; SMR000059035; CCRIS 764; SR-01000763435; NSC 7618; EINECS 200-563-4; EPA Pesticide Chemical Code 077902; BRN 0511852; sulfanilamine; Sulfanimide; Sulfanilamide [INN:DCF:NF]; AI3-00952; sulphanilic amide; 4-sulphanilamide; UNII-21240MF57M; Sulfanimide,(S); F-1162; Streptocid (TN); delta-Sulfanilamide; Prestwick_36; 4-sulfamoyl-aniline; sulfanilamide reagent; sulfanilamide-reagent; 4-sulphamoyl aniline; Spectrum_000489; 4-aminobenzensulfonamide; WLN: ZSWR DZ; 4-aminobenzenesulphonamide; p-aminobenzene sulfonamide; Prestwick0_000729; Prestwick1_000729; Prestwick2_000729; Prestwick3_000729; Spectrum2_000846; Spectrum3_001406; Spectrum4_000398; Spectrum5_001081; 4-aminobenzene sulfonamide; 4-aminobenzene-sulfonamide; p-aminosulfonyl phenylamine; SCHEMBL740; SULFANILAMIDE [MI]; 4-amino-benzenesulphonamide; Epitope ID:122232; AVC (TN); SULFANILAMIDE [HSDB]; Oprea1_273157; p-aminobenzene sulfonyl amide; BSPBio_000658; BSPBio_003052; KBioGR_000955; KBioSS_000969; SULFANILAMIDE [VANDF]; 4-14-00-02658 (Beilstein Handbook Reference); MLS001074682; MLS002152940; BIDD:GT0170; DivK1c_000528; SPECTRUM1500646; SULFANILAMIDE [MART.]; SULPHANILAMIDUM [HPUS]; SPBio_000831; SPBio_002597; SULFANILAMIDE [USP-RS]; SULFANILAMIDE [WHO-DD]; aromatic sulfonamide compound 5; BPBio1_000724; halogenosulfanilamide deriv. 5a; ZINC2101; DTXSID4023622; SCHEMBL11880061; BDBM10857; HMS501K10; KBio1_000528; KBio2_000969; KBio2_003537; KBio2_006105; KBio3_002272; Sulfanilamide, p.a., 99.0%; NINDS_000528; HMS1570A20; HMS1921O07; HMS2092E20; HMS2097A20; HMS2233B19; HMS3370J16; HMS3655K19; HMS3714A20; HMS3744M13; Pharmakon1600-01500646; Ro13354; SULFANILAMIDE [ORANGE BOOK]; HY-B0242; SULFANILAMIDE [EP MONOGRAPH]; Tox21_110351; Tox21_201331; Tox21_303336; AC9456; aromatic/heteroaromatic sulfonamide 2; BBL005257; c1264; CCG-40302; NSC757404; s1685; STK298902; UK-124; AKOS000119305; Tox21_110351_1; DB00259; NSC-757404; IDI1_000528; NCGC00016285-01; NCGC00016285-03; NCGC00016285-04; NCGC00016285-06; NCGC00016285-08; NCGC00091144-01; NCGC00091144-02; NCGC00091144-03; NCGC00257174-01; NCGC00258883-01; AS-13239; BP-12552; Sulfanilamide (4-Aminobenzenesulfonamide); SY009959; SBI-0051575.P002; AB00052138; FT-0657032; FT-0674702; S0381; SULFADIAZINE IMPURITY D [EP IMPURITY]; SW196353-3; EN300-16979; SULFADIMIDINE IMPURITY D [EP IMPURITY]; Sulfanilamide 1000 microg/mL in Acetonitrile; Sulfanilamide, JIS special grade, >=99.7%; Sulfanilamide, Vetec(TM) reagent grade, 97%; C07458; D08543; AB00052138-10; AB00052138_11; AB00052138_12; SULFADIMETHOXINE IMPURITY E [EP IMPURITY]; SULFAMETHOXAZOLE IMPURITY E [EP IMPURITY]; A834498; AC-907/25014139; Q423423; Sulfanilamide, VETRANAL(TM), analytical standard; SR-01000763435-2; SR-01000763435-3; SR-01000763435-4; SULFACETAMIDE SODIUM IMPURITY A [EP IMPURITY]; Sulfanilamide (166 degrees C) Melting Point Standard; Z56851240; F2190-0451; BENZENESULFONIC ACID,4-AMINO,AMIDE SULFANILAMIDE; Ethyl1-(aminomethyl)-6,8-dimethoxyisoquinoline-4-carboxylate; Sulfanilamide, European Pharmacopoeia (EP) Reference Standard; Sulfanilamide, United States Pharmacopeia (USP) Reference Standard; Reagecon Melting Point Sulphanilamide +164 to +166 degrees C Standard; SULFADIMETHOXINE SODIUM FOR VETERINARY USE IMPURITY E [EP IMPURITY]; Sulfanilamide Melting Point Standard, United States Pharmacopeia (USP) Reference Standard; 1337-39-9; Sulfanilamide melting point standard, Pharmaceutical Secondary Standard; Certified Reference Material
|