NPs Basic Information
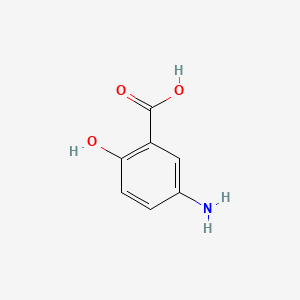
|
Name |
Mesalamine
|
| Molecular Formula | C7H7NO3 | |
| IUPAC Name* |
5-amino-2-hydroxybenzoic acid
|
|
| SMILES |
C1=CC(=C(C=C1N)C(=O)O)O
|
|
| InChI |
InChI=1S/C7H7NO3/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,9H,8H2,(H,10,11)
|
|
| InChIKey |
KBOPZPXVLCULAV-UHFFFAOYSA-N
|
|
| Synonyms |
5-Aminosalicylic acid; mesalamine; Mesalazine; 89-57-6; 5-Amino-2-hydroxybenzoic acid; Asacol; Pentasa; Canasa; 5-ASA; Claversal; Rowasa; Salofalk; m-Aminosalicylic acid; Lialda; Mesasal; Benzoic acid, 5-amino-2-hydroxy-; Fisalamine; Apriso; Lixacol; sfRowasa; p-Aminosalicylsaeure; Asacolitin; Mesalazina; Mesalazinum; Iialda; 5-amino-2-hydroxy-benzoic acid; Asacol HD; Mesalamine [USAN]; 5-Amino Salicylic Acid; SALICYLIC ACID, 5-AMINO-; 2-Hydroxy-5-aminobenzoic acid; MAX-002; 3-carboxy-4-hydroxyaniline; MFCD00007877; NSC 38877; NSC-38877; Mesalazine [INN]; MLS001424012; 4Q81I59GXC; CHEBI:6775; mesalamine (USAN); 5-?Aminosalicylic Acid (Mesalazine); CAS-89-57-6; NCGC00016344-03; Mesalazinum [Latin]; SMR000145728; DSSTox_CID_4506; DSSTox_RID_77435; DSSTox_GSID_24506; Mesalazina [Spanish]; p-Aminosalicylsaeure [German]; Mesavancol; Delzicol; Mesavance; Mezavant; Mesalazine MMX; Mezavant XL; Mesalamine (USP); Pentasa (TN); Salofalk Granu-Stix; Apriso (TN); Asacol (TN); Canasa (TN); Lialda (TN); Rowasa (TN); 5-AS; CCRIS 7334; SR-01000763486; Mesalamine [USAN:USP]; EINECS 201-919-1; BRN 2090421; UNII-4Q81I59GXC; AI3-15564; HSDB 7512; AJG-501; SPD 476; SPD-476; SPD-480; Mesalamine (TN); Delzicol (TN); Sfrowasa (TN); Mesalamine (Lialda); 5-aminosalicylic_acid; MD-0901; 5-Aminosalicyclic acid; 5-amino-salicylic acid; MESALAMINE [MI]; MESALAZINE [JAN]; Prestwick0_001069; Prestwick1_001069; Prestwick2_001069; Prestwick3_001069; MESALAMINE [HSDB]; WLN: ZR DQ CVQ; Z-206; MESALAMINE [VANDF]; CHEMBL704; Mesalazine (JP17/INN); EC 201-919-1; cid_4075; MESALAZINE [MART.]; MESALAMINE [USP-RS]; MESALAZINE [WHO-DD]; Oprea1_847633; SCHEMBL31297; 3amino-6-hydroxybenzoic acid; BSPBio_001058; KBioGR_002425; KBioSS_002431; 4-14-00-02058 (Beilstein Handbook Reference); MLS000758287; 5-Aminosalicylic acid, 95%; 5-Aminosalicylic acid, tablet; BIDD:GT0811; 3-amino-6-hydroxybenzoic acid; SPBio_002969; BPBio1_001164; GTPL2700; ZINC1688; 5-Amino 2-hydroxy benzoic acid; DTXSID5024506; MESALAMINE [ORANGE BOOK]; MESALAZINE [EP IMPURITY]; SCHEMBL18038934; 5-Aminosalicylic acid, >=99%; BDBM60918; KBio2_002425; KBio2_004993; KBio2_007561; KBio3_002904; MESALAZINE [EP MONOGRAPH]; cMAP_000045; HMS1571E20; HMS2051M21; HMS2090I09; HMS2098E20; HMS3393M21; HMS3649K15; HMS3651M15; HMS3715E20; MESALAMINE [USP MONOGRAPH]; Pharmakon1600-01505993; BCP05326; NSC38877; Tox21_110384; Tox21_201610; Tox21_303125; AC8101; BBL013046; NSC759301; s1681; STK301678; AKOS000118959; Tox21_110384_1; AC-2764; BCP9000175; CCG-100829; DB00244; HS-0100; NC00079; NSC-759301; NCGC00016344-01; NCGC00016344-02; NCGC00016344-04; NCGC00016344-05; NCGC00016344-07; NCGC00090934-01; NCGC00090934-02; NCGC00257142-01; NCGC00259159-01; 5-amino-2-hydroxobenzoic acid monohydrate; BP-13074; HY-15027; SY002854; 5-Aminosalicylic acid, analytical standard; A0317; AB00374979; AM20060091; FT-0619950; SW197303-4; EN300-18389; C07138; D00377; AB00374979-09; AB00374979-10; AB00374979_11; AB00374979_12; Q412479; 5-amino-2-hydroxybenzoic acid,5-Aminosalicylic acid; Q-201355; SR-01000763486-3; SR-01000763486-4; SR-01000763486-9; Z57127471; F1918-0003; Mesalazine, European Pharmacopoeia (EP) Reference Standard; Mesalamine, United States Pharmacopeia (USP) Reference Standard; Mesalamine, Pharmaceutical Secondary Standard; Certified Reference Material; Mesalazine for system suitability, European Pharmacopoeia (EP) Reference Standard; 51481-17-5
|
|
| CAS | 89-57-6 | |
| PubChem CID | 4075 | |
| ChEMBL ID | CHEMBL704 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 153.14 | ALogp: | 1.3 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.416 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.407 | MDCK Permeability: | 0.00000813 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.925 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.996 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.146 | Plasma Protein Binding (PPB): | 51.10% |
| Volume Distribution (VD): | 0.372 | Fu: | 66.74% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.032 | CYP1A2-substrate: | 0.075 |
| CYP2C19-inhibitor: | 0.059 | CYP2C19-substrate: | 0.049 |
| CYP2C9-inhibitor: | 0.288 | CYP2C9-substrate: | 0.104 |
| CYP2D6-inhibitor: | 0.018 | CYP2D6-substrate: | 0.146 |
| CYP3A4-inhibitor: | 0.048 | CYP3A4-substrate: | 0.061 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.193 | Half-life (T1/2): | 0.829 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.021 | Human Hepatotoxicity (H-HT): | 0.555 |
| Drug-inuced Liver Injury (DILI): | 0.843 | AMES Toxicity: | 0.035 |
| Rat Oral Acute Toxicity: | 0.051 | Maximum Recommended Daily Dose: | 0.007 |
| Skin Sensitization: | 0.47 | Carcinogencity: | 0.062 |
| Eye Corrosion: | 0.02 | Eye Irritation: | 0.964 |
| Respiratory Toxicity: | 0.969 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000097 | 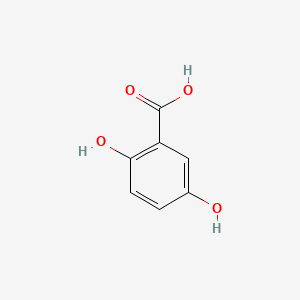 |
0.657 | D0C4YC | 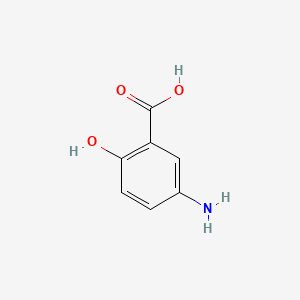 |
1.000 | ||
| ENC001108 | 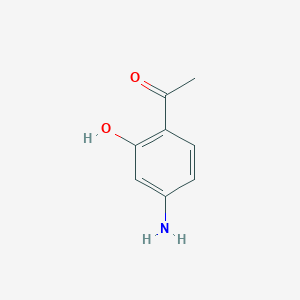 |
0.568 | D01WJL | 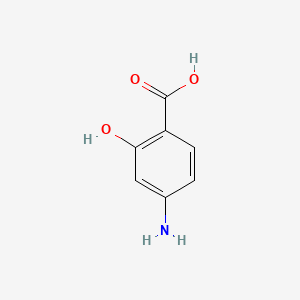 |
0.758 | ||
| ENC000069 | 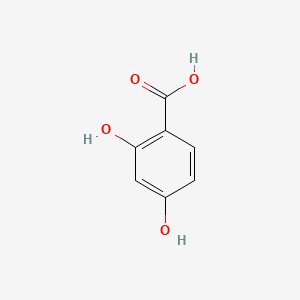 |
0.526 | D0S2BT | 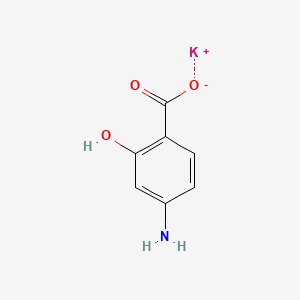 |
0.553 | ||
| ENC000002 | 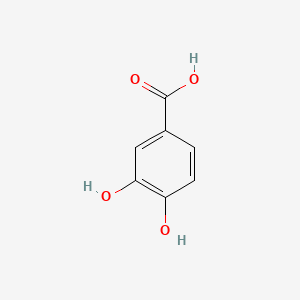 |
0.487 | D07HBX |  |
0.514 | ||
| ENC000344 | 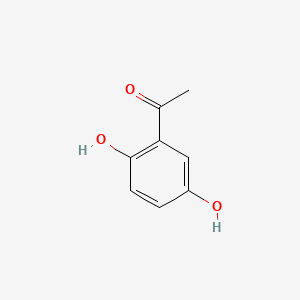 |
0.487 | D08LFZ | 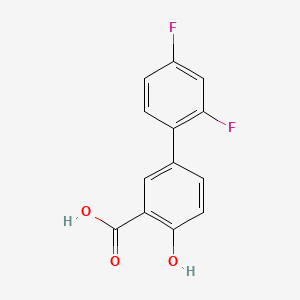 |
0.418 | ||
| ENC000296 | 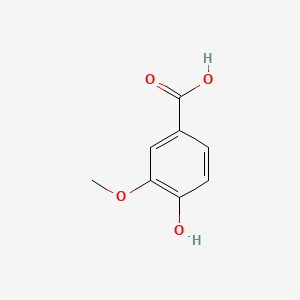 |
0.452 | D08HVR | 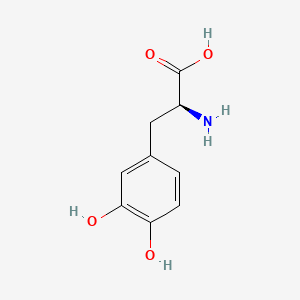 |
0.404 | ||
| ENC002913 | 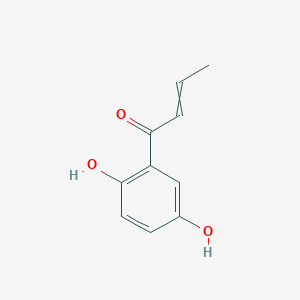 |
0.422 | D0V9EN |  |
0.391 | ||
| ENC000035 | 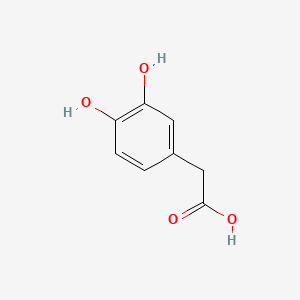 |
0.419 | D0I3RO | 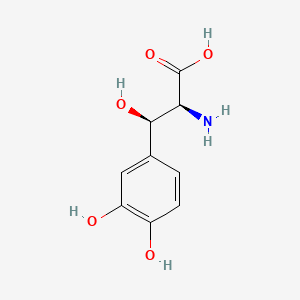 |
0.388 | ||
| ENC001030 | 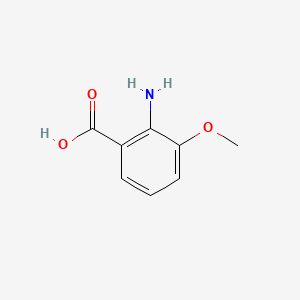 |
0.419 | D0BA6T | 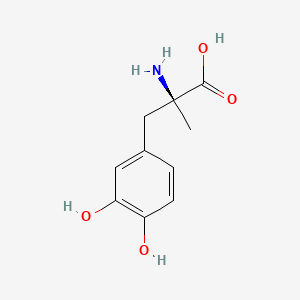 |
0.388 | ||
| ENC000862 | 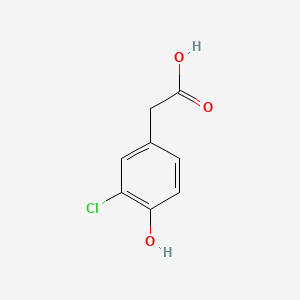 |
0.419 | D0L5PO | 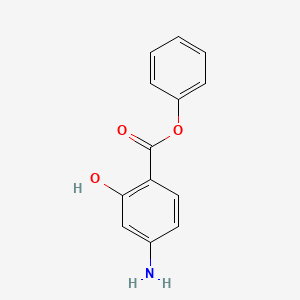 |
0.375 | ||