NPs Basic Information
|
Name |
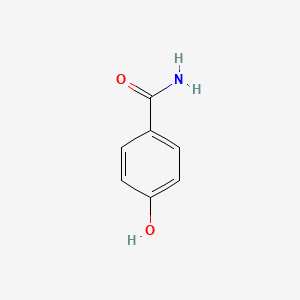
4-Hydroxybenzamide
|
| Molecular Formula | C7H7NO2 | |
| IUPAC Name* |
4-hydroxybenzamide
|
|
| SMILES |
C1=CC(=CC=C1C(=O)N)O
|
|
| InChI |
InChI=1S/C7H7NO2/c8-7(10)5-1-3-6(9)4-2-5/h1-4,9H,(H2,8,10)
|
|
| InChIKey |
QXSAKPUBHTZHKW-UHFFFAOYSA-N
|
|
| Synonyms |
4-Hydroxybenzamide; 619-57-8; p-Hydroxybenzamide; Benzamide, 4-hydroxy-; 4-Hydroxy-benzamide; MFCD00007997; 9OU5YD093J; CHEMBL123617; NSC-524134; 41911-58-4; UNII-9OU5YD093J; 4-carbamoylphenol; EINECS 210-602-7; NSC 524134; para-hydroxybenzoic amide; AI3-15540; para-hydroxy benzoic amide; 4-Hydroxybenzamide, 98%; 4-hydroxybenzene carboxamide; Oprea1_692786; BENZAMIDE, P-HYDROXY-; SCHEMBL162830; SCHEMBL10792133; DTXSID10210931; P-HYDROXYBENZOIC ACID AMIDE; ZINC157088; AMY16513; 4-hydroxybenzamide 41911-58-4; 4-HYDROXY-BENZOIC ACID,AMIDE; BDBM50106201; NSC524134; AKOS000121433; DS-9435; NCGC00341573-01; DB-054032; FT-0618687; H0200; EN300-18541; D90813; AB00443728-03; A833505; AE-562/40219003; W-105070; Q27272839; Z104502228
|
|
| CAS | 619-57-8 | |
| PubChem CID | 65052 | |
| ChEMBL ID | CHEMBL123617 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 137.14 | ALogp: | 0.3 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 63.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.618 | MDCK Permeability: | 0.00000989 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.99 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.009 |
| 30% Bioavailability (F30%): | 0.887 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.901 | Plasma Protein Binding (PPB): | 53.92% |
| Volume Distribution (VD): | 0.99 | Fu: | 64.85% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.366 | CYP1A2-substrate: | 0.174 |
| CYP2C19-inhibitor: | 0.072 | CYP2C19-substrate: | 0.059 |
| CYP2C9-inhibitor: | 0.033 | CYP2C9-substrate: | 0.638 |
| CYP2D6-inhibitor: | 0.024 | CYP2D6-substrate: | 0.47 |
| CYP3A4-inhibitor: | 0.021 | CYP3A4-substrate: | 0.162 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.435 | Half-life (T1/2): | 0.543 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.077 | Human Hepatotoxicity (H-HT): | 0.069 |
| Drug-inuced Liver Injury (DILI): | 0.386 | AMES Toxicity: | 0.014 |
| Rat Oral Acute Toxicity: | 0.298 | Maximum Recommended Daily Dose: | 0.009 |
| Skin Sensitization: | 0.168 | Carcinogencity: | 0.295 |
| Eye Corrosion: | 0.011 | Eye Irritation: | 0.981 |
| Respiratory Toxicity: | 0.085 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000007 | 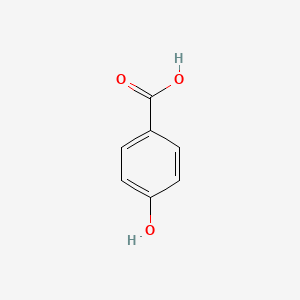 |
0.688 | D0U5QK | 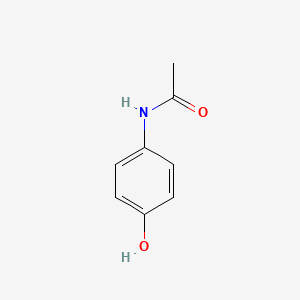 |
0.500 | ||
| ENC000200 | 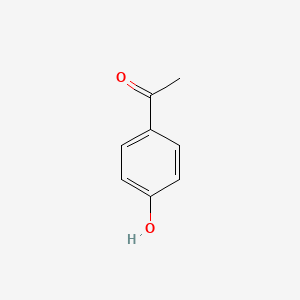 |
0.688 | D03UOT |  |
0.485 | ||
| ENC000195 | 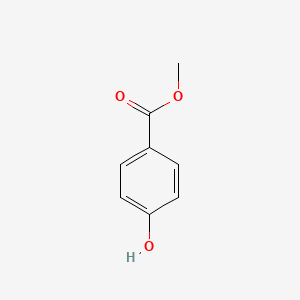 |
0.629 | D01CRB | 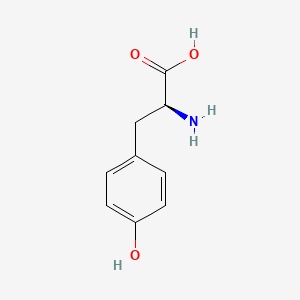 |
0.476 | ||
| ENC000774 | 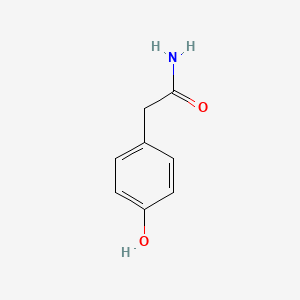 |
0.583 | D0B3QM | 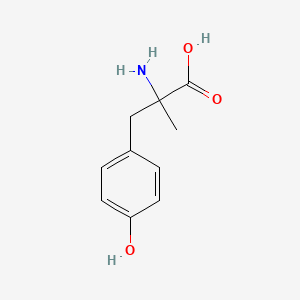 |
0.455 | ||
| ENC005097 | 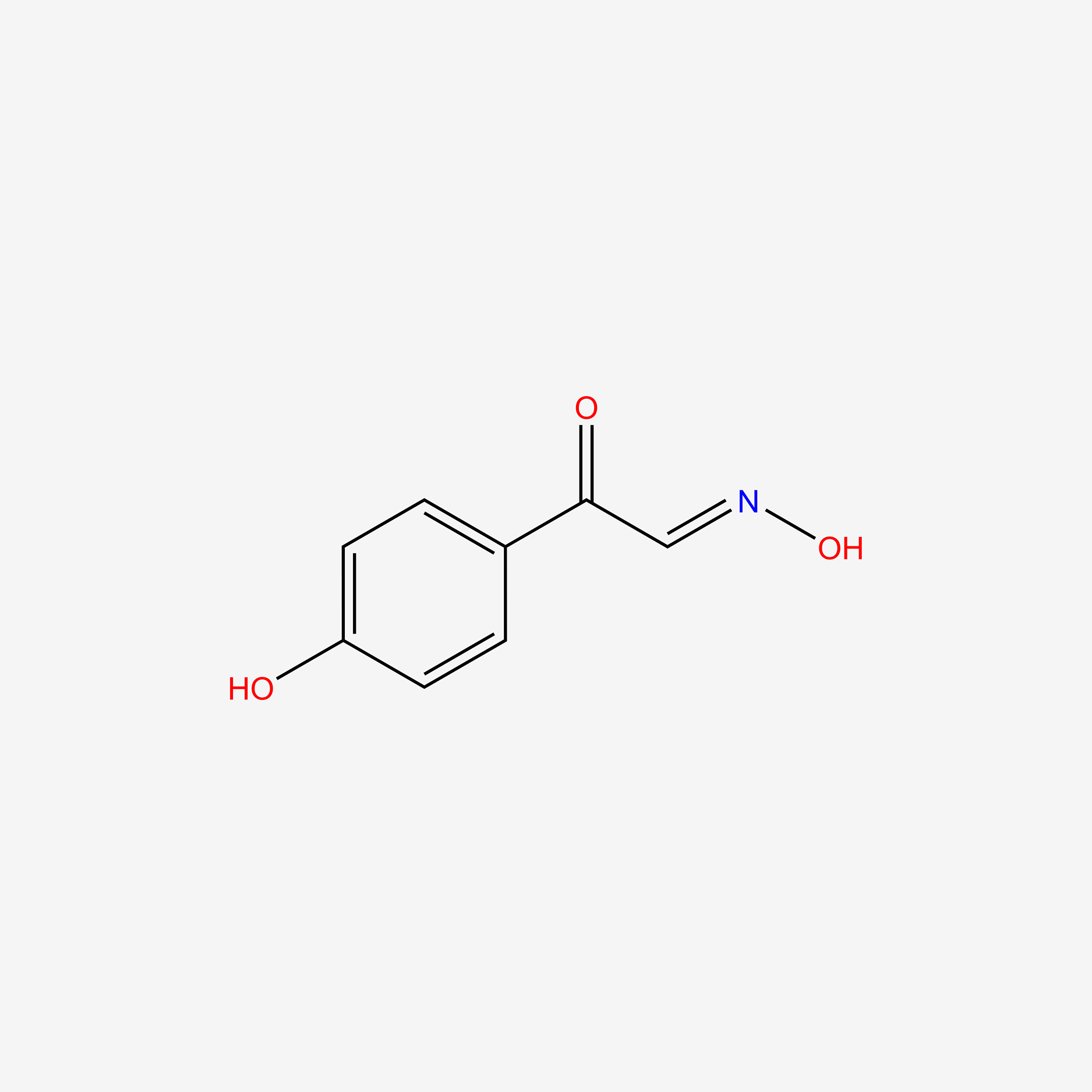 |
0.579 | D02WAB | 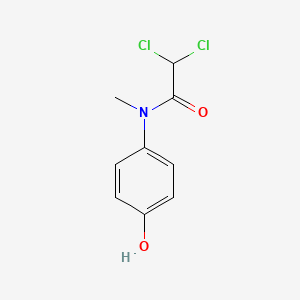 |
0.422 | ||
| ENC001049 | 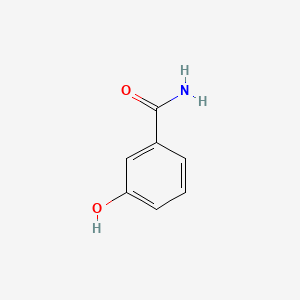 |
0.543 | D0W1RY | 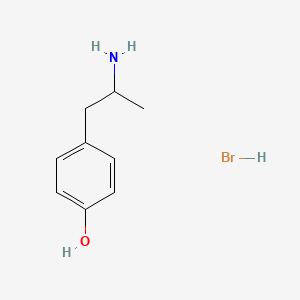 |
0.415 | ||
| ENC000006 | 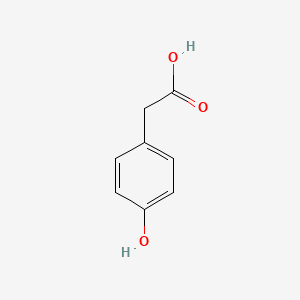 |
0.500 | D0Q8ZX | 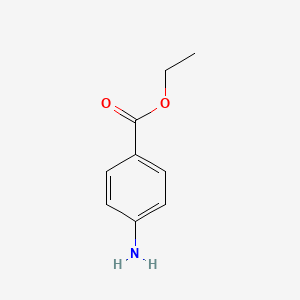 |
0.395 | ||
| ENC000072 | 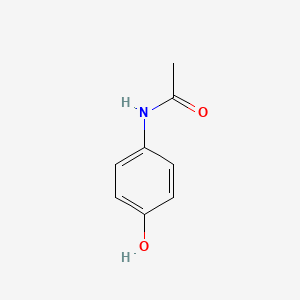 |
0.500 | D06OAV | 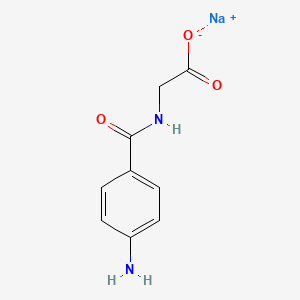 |
0.347 | ||
| ENC000005 | 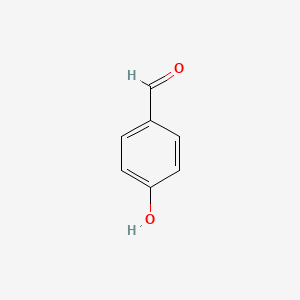 |
0.486 | D0S2BV | 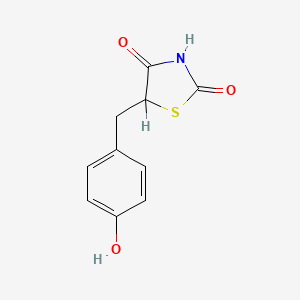 |
0.327 | ||
| ENC000086 | 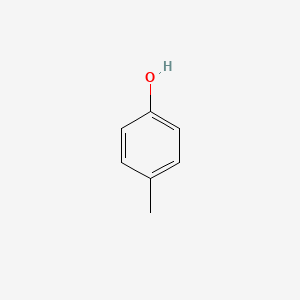 |
0.485 | D09ZQN | 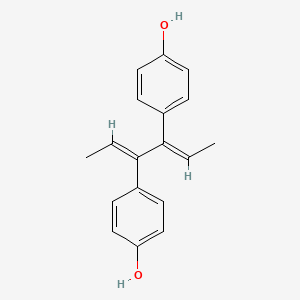 |
0.317 | ||