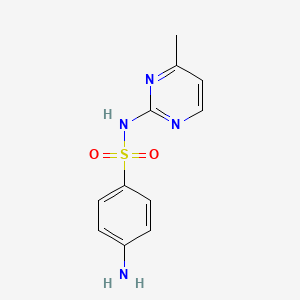
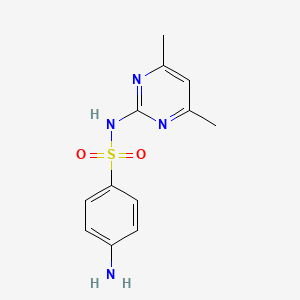
| InChI |
InChI=1S/C11H12N4O2S/c1-8-6-7-13-11(14-8)15-18(16,17)10-4-2-9(12)3-5-10/h2-7H,12H2,1H3,(H,13,14,15)
|
| Synonyms |
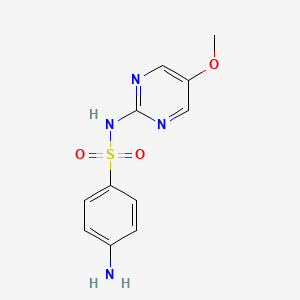
sulfamerazine; 127-79-7; Sulphamerazine; Sulfamerazin; Sulfamethyldiazine; Cremomerazine; Methylsulfazine; Sulfameradine; Mebacid; Mesulfa; Metilsulfadiazin; 2-Sulfa-4-methylpyrimidine; 4-amino-N-(4-methylpyrimidin-2-yl)benzenesulfonamide; Methylpyrimal; Metilsulfazin; Sulfamerazina; Sulfamerazinum; Kelamerazine; Percoccide; Pyralcid; Septacil; Septosyl; Solumedin; Sumedine; Romezin; Veta-Merazine; Debenal-M; Pirimal-M; Pyrimal M; Methylsulfazin; Benzenesulfonamide, 4-amino-N-(4-methyl-2-pyrimidinyl)-; N-(4-Methyl-2-pyrimidyl)sulfanilamide; 2-(Sulfanilamido)-4-methylpyrimidine; 2-(4-Aminobenzenesulfonamido)-4-methylpyrimidine; 4-Amino-N-(4-methyl-2-pyrimidinyl)-benzenesulfonamide; 2643-RP; RP 2632; N1-(4-Methyl-2-pyrimidinyl)sulfanilamide; (p-Aminobenzolsulfonyl)-2-amino-4-methylpyrimidin; A-310; 4-Amino-N-(4-methyl-2-pyrimidinyl)benzenesulfonamide; NSC 27259; RP2632; Sulfanilamide, N1-(4-methyl-2-pyrimidinyl)-; Solumedine; Sulfamerazine (INN); CHEBI:102130; 2-Sulfanilamido-4-methylpyrimidine; 2632 R. P.; N(1)-(4-Methyl-2-pyrimidinyl)sulfanilamide; 4-amino-N-(4-methylpyrimidin-2-yl)benzene-1-sulfonamide; 2-(p-Aminobenzolsulfonamido)-4-methylpyrimidine; NSC-27259; UR1SAB295F; MLS000551747; Solfamerazina; Sulfanilamide, N(sup 1)-(4-methyl-2-pyrimidinyl)-; NCGC00016386-01; CAS-127-79-7; SMR000145672; Solfamerazina [DCIT]; Sulfamerazine 100 microg/mL in Acetonitrile; DSSTox_CID_3612; SULFAMERAZINE [INN]; DSSTox_RID_77109; DSSTox_GSID_23612; Sulfamerazinum [INN-Latin]; Sulfamerazina [INN-Spanish]; 4-Amino-N-(4-methyl-pyrimidin-2-yl)-benzenesulfonamide; 4-amino-N-(4-methylpyrimidin-2-yl); SR-01000684857; EINECS 204-866-2; N(sup 1)-(4-Methyl-2-pyrimidinyl)sulfanilamide; UNII-UR1SAB295F; BRN 0249133; AI3-08026; Sulfamerazine [USP:INN:BAN]; (p-Aminobenzolsulfonyl)-2-amino-4-methylpyrimidin [German]; Prestwick_17; MFCD00023212; Sulfamerazine-13C6; Spectrum_000003; N1-(4-Methylpyrimidin-2-yl)sulfanilamide; Opera_ID_988; Prestwick0_000694; Prestwick1_000694; Prestwick2_000694; Prestwick3_000694; Spectrum2_001320; Spectrum3_001363; Spectrum4_000343; Spectrum5_001413; 2(p-Aminobenzolsulfonamido)-4-methylpyrimidin; SULFAMERAZINE [MI]; CHEMBL438; Epitope ID:122236; N(sup1)-(4-Methyl-2-pyrimidinyl)sulfanilamide; SCHEMBL33999; BSPBio_000847; BSPBio_002886; KBioGR_000745; KBioSS_000343; SULFAMERAZINE [VANDF]; 5-25-10-00167 (Beilstein Handbook Reference); MLS001201765; DivK1c_000563; SPECTRUM1500547; SULFAMERAZINE [MART.]; SPBio_001419; SPBio_002768; SULFAMERAZINE [USP-RS]; SULFAMERAZINE [WHO-DD]; BPBio1_000933; DTXSID0023612; HMS501M05; KBio1_000563; KBio2_000343; KBio2_002911; KBio2_005479; KBio3_002106; ZINC57501; NINDS_000563; HMS1570K09; HMS1921A15; HMS2092I17; HMS2097K09; HMS2234D16; HMS3374K04; HMS3652I03; HMS3714K09; Pharmakon1600-01500547; SULFAMERAZINE [GREEN BOOK]; SULFAMERAZINE [ORANGE BOOK]; ALBB-025702; AMY23374; HY-B0512; NSC27259; SULFAMERAZINE [EP MONOGRAPH]; Tox21_110411; BBL003544; CCG-39258; NSC757325; s3132; STK520614; SULFOSE COMPONENT SULFAMERAZINE; AKOS005143010; TERFONYL COMPONENT SULFAMERAZINE; Tox21_110411_1; DB01581; KS-5323; NSC-757325; IDI1_000563; LANTRISUL COMPONENT SULFAMERAZINE; SULFALOID COMPONENT SULFAMERAZINE; NCGC00016386-02; NCGC00016386-03; NCGC00016386-06; NCGC00094787-01; NCGC00094787-02; NEOTRIZINE COMPONENT SULFAMERAZINE; SULFAMERAZINE COMPONENT OF SULFOSE; N'-(4-Methyl-2-pyrimidyl) sulfanilamide; SULFAMERAZINE (TRISULFAPYRIMIDINES); SULFAMERAZINE COMPONENT OF TERFONYL; TRISULFAPYRIMIDINES (SULFAMERAZINE); SBI-0051521.P003; SULFAMERAZINE COMPONENT OF LANTRISUL; SULFAMERAZINE COMPONENT OF SULFALOID; DB-041873; SULFAMERAZINE COMPONENT OF NEOTRIZINE; Sulfamerazine, ReagentPlus(R), >=99.0%; AB00052096; FT-0631745; FT-0645132; SW196334-3; TRIPLE SULFOID COMPONENT SULFAMERAZINE; SULFADIMIDINE IMPURITY A [EP IMPURITY]; Sulfamerazine 1000 microg/mL in Acetonitrile; Sulfamerazine, Vetec(TM) reagent grade, 98%; 2-(p-Aminobenzosulfonamido)-4-methylpyrimidine; D02435; D84140; EN300-202655; AB00052096-13; AB00052096_15; AB00052096_16; SULFAMERAZINE COMPONENT OF TRIPLE SULFOID; A805747; Q415196; Sulfamerazine, VETRANAL(TM), analytical standard; Q-201761; SR-01000684857-2; SR-01000684857-4; BRD-K93524252-001-05-6; BRD-K93524252-001-15-5; F2190-0484; N(SUP 1)-(4-METHYL-2- PYRIMIDINYL)SULFANILAMIDE; TRISULFAPYRIMIDINES (SULFAMERAZINE) [ORANGE BOOK]; Z1954804578; Sulfamerazine, European Pharmacopoeia (EP) Reference Standard; Sulfamerazine, United States Pharmacopeia (USP) Reference Standard; Sulfamerazine, Pharmaceutical Secondary Standard; Certified Reference Material
|