| InChI |
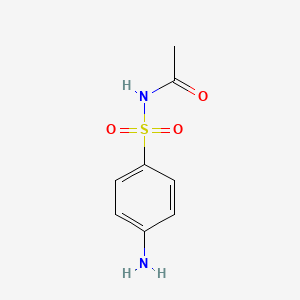
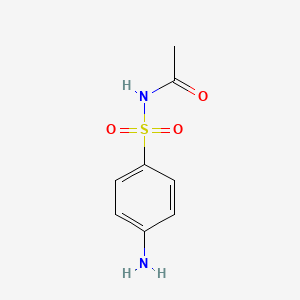
InChI=1S/C8H10N2O3S/c1-6(11)10-14(12,13)8-4-2-7(9)3-5-8/h2-5H,9H2,1H3,(H,10,11)
|
| Synonyms |
sulfacetamide; 144-80-9; Sulphacetamide; Acetosulfamine; N-((4-Aminophenyl)sulfonyl)acetamide; N-Acetylsulfanilamide; N-Sulfanilylacetamide; Albucid; Acetosulfamin; Urosulfon; N-Sulphanilylacetamide; Sulfacetimide; Acetocid; Sulfacet; Sulfacyl; Sulfanilacetamide; Formosulfacetamide; N-(4-aminophenyl)sulfonylacetamide; Albamine; Sulphasil; Urosulfone; Alesten; Oclucid; Region; Sulamyd; Sulfanilazetamid; p-Aminobenzenesulfonacetamide; Sulf-10; Bleph-10; N-[(4-Aminophenyl)sulfonyl]acetamide; N-Acetyl-4-aminobenzenesulfonamide; Sulfacetamidum; Sulphacetamidum; Steramide; Sebizon; Isopto cetamide; Ophthel-S; p-Aminobenzenesulfonoacetamide; N'-Acetylsulfanilamide; Sulfacetamida; Bleph-10 liquifilm; OP-Sulfa 30; Sulfacylum; Sulfacel-15; Acetamide, N-sulfanilyl-; Klaron; N-((p-Aminophenyl)sulfonyl)acetamide; N(sup1)-Acetylsulfanilamide; A-500; Ocusulf-10; Sulfair-15; Sulten-10; N-(4-Aminophenylsulfonyl)acetamide; SULSTER; Sulf-15; N-[(p-Aminophenyl)sulfonyl]acetamide; FML-S; N(sup 1)-Acetyl-4-aminophenylsulfonamide; Sulfanilamide, N(sup 1)-acetyl-; Acetamide, N-((4-aminophenyl)sulfonyl)-; Acetamide, N-[(4-aminophenyl)sulfonyl]-; NSC 63871; N-(p-Aminobenzenesulfonyl)acetamide; N(1)-acetylsulfanilamide; MFCD00066501; NSC-63871; CHEMBL455; Triple sulfa (sulfacetamide); Metimyd; MLS000069710; Solfacetamide; CHEBI:63845; OCUSULF-30; BLEPH-30; N-acetyl-4-amino-benzenesulfonamide; N(1)-acetyl-4-aminophenylsulfonamide; 4965G3J0F5; Sulfacyl (VAN); NCGC00018242-04; NCGC00018242-07; SMR000058173; N1-Acetylsulfanilamide; Solfacetamide [DCIT]; Sulfacetamide 100 microg/mL in Acetonitrile; DSSTox_CID_6060; 5414-91-5; DSSTox_RID_78000; Sulfanilazetamid [German]; DSSTox_GSID_26060; N-Acetylsulfanilamine; Sulfacetamidum [INN-Latin]; N-(4-Aminobenzenesulfonyl)acetamide; Sulfacetamida [INN-Spanish]; N(sup 1)-Acetylsulfanilamide; Caswell No. 808A; CAS-144-80-9; CCRIS 6273; EINECS 205-640-6; Sulfacetamide (USP/INN); EPA Pesticide Chemical Code 077904; BRN 0981718; AI3-26837; Sulfacetamide [USP:INN:BAN]; UNII-4965G3J0F5; Sulfacetamide, 1; CETAPRED; PREDAMIDE; Spectrum_000984; Opera_ID_1990; Prestwick0_000014; Prestwick1_000014; Prestwick2_000014; Prestwick3_000014; Spectrum2_001318; Spectrum3_001017; Spectrum4_001146; Spectrum5_001230; WLN: ZR DSWMV1; SULFACETAMIDE [MI]; CBChromo1_000180; Sulfanilamide, N1-acetyl-; 4-acetylaminosulfonylaniline; SULFACETAMIDE [INN]; NCIOpen2_002838; Sulfacetamide, >=98.0%; Oprea1_686524; Oprea1_810285; SCHEMBL40863; BSPBio_000047; BSPBio_002773; CBDivE_013934; KBioGR_001691; KBioSS_001464; SULFACETAMIDE [VANDF]; 4-14-00-02662 (Beilstein Handbook Reference); MLS001076499; DivK1c_000227; SPECTRUM1500545; SULFACETAMIDE [MART.]; SPBio_001415; SPBio_001968; SULFACETAMIDE [WHO-DD]; SULFACETAMIDE [WHO-IP]; BPBio1_000053; DTXSID8026060; HMS500L09; KBio1_000227; KBio2_001464; KBio2_004032; KBio2_006600; KBio3_001993; 4-[(acetylamino)sulfonyl]aniline; NINDS_000227; 4-[(acetylamino) sulfonyl]aniline; HMS1921A11; HMS2092I13; HMS2232K07; HMS3259G06; HMS3374O02; Pharmakon1600-01500545; SULFACETAMIDE [ORANGE BOOK]; Sulfacetamide, >=98.0% (NT); HY-N7123; NSC63871; ZINC5179119; SULFACETAMIDE (TRIPLE SULFA); SULFACETAMIDE [USP IMPURITY]; Tox21_110846; Tox21_201999; Tox21_303100; AC2473; BBL012083; BDBM50316126; CCG-39256; NSC757323; s5546; STK068185; TRYSUL COMPONENT SULFACETAMIDE; SULFACETAMIDUM [WHO-IP LATIN]; SULTRIN COMPONENT SULFACETAMIDE; VAGILIA COMPONENT SULFACETAMIDE; AKOS000119729; N-[(4-aminobenzene)sulfonyl]acetamide; Tox21_110846_1; AC-7630; DB00634; KS-5327; NC00538; NSC-757323; GYNE-SULF COMPONENT SULFACETAMIDE; IDI1_000227; SULFACETAMIDE COMPONENT OF TRYSUL; NCGC00018242-01; NCGC00018242-02; NCGC00018242-03; NCGC00018242-05; NCGC00018242-06; NCGC00018242-08; NCGC00021857-03; NCGC00021857-04; NCGC00021857-05; NCGC00257159-01; NCGC00259548-01; SULFACETAMIDE COMPONENT OF SULTRIN; SULFACETAMIDE COMPONENT OF VAGILIA; SY014752; SBI-0051519.P003; DB-042749; SULFACETAMIDE COMPONENT OF GYNE-SULF; CS-0013812; FT-0631838; S0577; EN300-18138; SULFADIMIDINE IMPURITY E [EP IMPURITY]; D05947; AB00052094_14; SR-01000000194; Sulfacetamide, VETRANAL(TM), analytical standard; TRIPLE SULFA (SULFACETAMIDE) [ORANGE BOOK]; Q-201757; Q2022983; SR-01000000194-2; Sulfacetamide, Antibiotic for Culture Media Use Only; BRD-K21520694-001-12-3; N-((4-AMINOPHENYL)SULFONYL)ACETAMIDE [WHO-IP]; Z57202572; Sulfacetamide, United States Pharmacopeia (USP) Reference Standard
|