NPs Basic Information
|
Name |
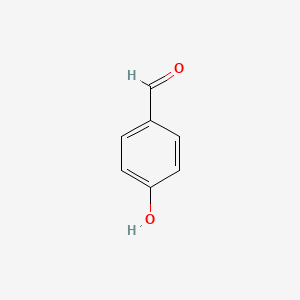
4-Hydroxybenzaldehyde
|
| Molecular Formula | C7H6O2 | |
| IUPAC Name* |
4-hydroxybenzaldehyde
|
|
| SMILES |
C1=CC(=CC=C1C=O)O
|
|
| InChI |
InChI=1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H
|
|
| InChIKey |
RGHHSNMVTDWUBI-UHFFFAOYSA-N
|
|
| Synonyms |
4-hydroxybenzaldehyde; p-Hydroxybenzaldehyde; 123-08-0; 4-Formylphenol; p-Formylphenol; p-Oxybenzaldehyde; Benzaldehyde, 4-hydroxy-; Parahydroxybenzaldehyde; Benzaldehyde, p-hydroxy-; 4-HYDROXY-BENZALDEHYDE; 4-Hydroxy benzaldehyde; USAF M-6; MFCD00006939; 4-formyl phenol; NSC 2127; p-Hydroxy-benzaldehyde; Para-Hydroxybenzaldehyde; 4-Hydroxybenzenecarbonal; CHEMBL14193; CHEBI:17597; NSC-2127; O1738X3Y38; EINECS 204-599-1; BRN 0471352; AI3-15366; CCRIS 8911; PARA-HYDROXY BENZALDEHYDE; 4-formyl-phenol; UNII-O1738X3Y38; 4hydroxybenzaldehyde; p-hydroxibezaldehyde; p-hydroxybenzaldehye; 4-hydoxybenzaldehyde; 4-hydroxybenzaldehyd; 4-hydroxybezaldehyde; 4-Hydroxybenzaldehye; 4-hydroxibenzaldehyde; 4-hydroxylbenzaldehyde; p-hydroxy benzaldehyde; 4--hydroxybenzaldehyde; p-hydroxyl benzaldehyde; 4-hydroxyl benzaldehyde; 4-hydroxy- benzaldehyde; WLN: VHR DQ; bmse000259; bmse000582; bmse010005; SCHEMBL37193; 4-Hydroxybenzaldehyde, 98%; 4-08-00-00251 (Beilstein Handbook Reference); BIDD:ER0339; p-Hydroxybenzaldehyde, Pract.; 4-Hydroxybenzaldehyde, >=97%; DTXSID8059552; FEMA NO. 3984; HYDROXYBENZALDEHYDE [INCI]; NSC2127; 1k03; P-HYDROXYBENZALDEHYDE [MI]; ZINC156709; ACT01156; BCP26952; CS-D1179; HY-Y0313; STR00705; 4-HYDROXYBENZALDEHYDE [FHFI]; 4-Hydroxybenzaldehyde, >=97%, FG; BDBM50177411; BR1235; s6008; STK188428; AKOS000119184; AC-2984; AM10667; DB03560; PS-3635; NCGC00188243-01; NCGC00188243-02; 4-Hydroxybenzaldehyde, analytical standard; 65581-83-1; BP-30158; SY003489; 4-Hydroxybenzaldehyde, >=95.0% (HPLC); DB-003763; FT-0631713; FT-0669408; H0198; EN300-18030; 4-(Hydroxymethylene)-2,5-cyclohexadiene-1-one; C00633; H-3800; AB-131/40191192; BISOPROLOL FUMARATE IMPURITY S [EP IMPURITY]; Q1953888; 4-Hydroxybenzaldehyde, Vetec(TM) reagent grade, 95%; Z57127520; F2190-0635; p-Hydroxybenzaldehyde;4-Formylphenol;p-Formylphenol;Bisoprolol Fumarate EP Impurity S
|
|
| CAS | 123-08-0 | |
| PubChem CID | 126 | |
| ChEMBL ID | CHEMBL14193 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 122.12 | ALogp: | 1.4 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.575 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.451 | MDCK Permeability: | 0.00001250 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.01 | 20% Bioavailability (F20%): | 0.21 |
| 30% Bioavailability (F30%): | 0.302 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.307 | Plasma Protein Binding (PPB): | 61.79% |
| Volume Distribution (VD): | 0.895 | Fu: | 37.42% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.793 | CYP1A2-substrate: | 0.089 |
| CYP2C19-inhibitor: | 0.119 | CYP2C19-substrate: | 0.072 |
| CYP2C9-inhibitor: | 0.03 | CYP2C9-substrate: | 0.862 |
| CYP2D6-inhibitor: | 0.013 | CYP2D6-substrate: | 0.456 |
| CYP3A4-inhibitor: | 0.053 | CYP3A4-substrate: | 0.227 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.131 | Half-life (T1/2): | 0.872 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.033 | Human Hepatotoxicity (H-HT): | 0.03 |
| Drug-inuced Liver Injury (DILI): | 0.03 | AMES Toxicity: | 0.046 |
| Rat Oral Acute Toxicity: | 0.054 | Maximum Recommended Daily Dose: | 0.03 |
| Skin Sensitization: | 0.393 | Carcinogencity: | 0.214 |
| Eye Corrosion: | 0.986 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.973 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001854 |  |
0.595 | D03UOT |  |
0.516 | ||
| ENC001420 |  |
0.568 | D0U5QK |  |
0.447 | ||
| ENC000026 | 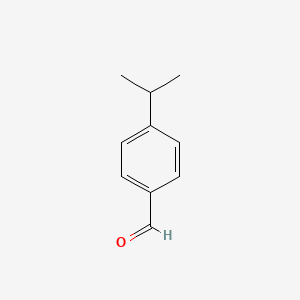 |
0.528 | D0E9CD |  |
0.410 | ||
| ENC000034 | 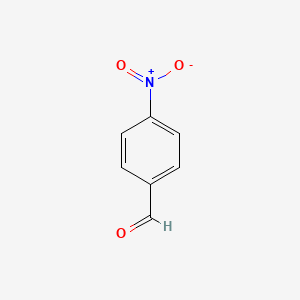 |
0.528 | D0W1RY | 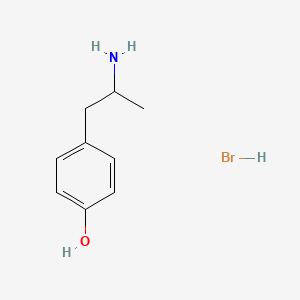 |
0.400 | ||
| ENC001021 | 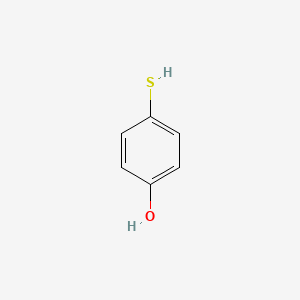 |
0.516 | D01CRB | 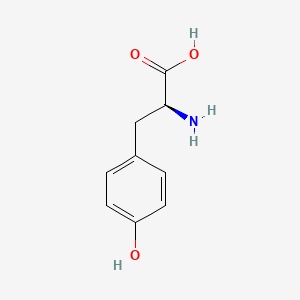 |
0.395 | ||
| ENC000086 | 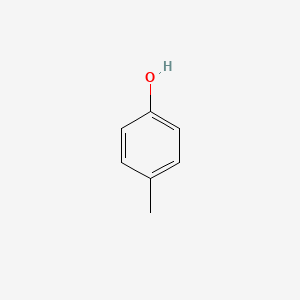 |
0.516 | D02WAB | 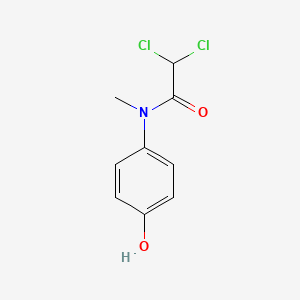 |
0.378 | ||
| ENC000665 | 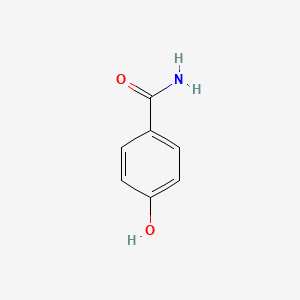 |
0.486 | D0B3QM | 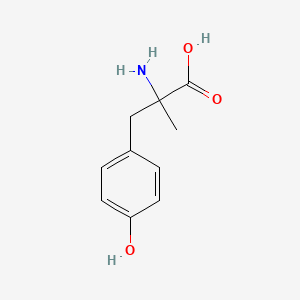 |
0.378 | ||
| ENC000007 | 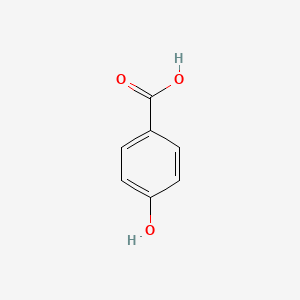 |
0.486 | D0S2BV | 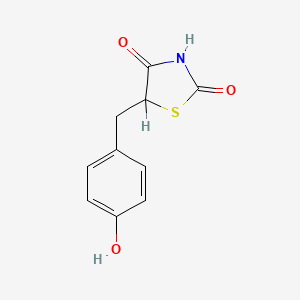 |
0.340 | ||
| ENC000200 | 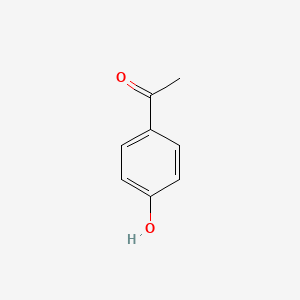 |
0.486 | D0H6TP | 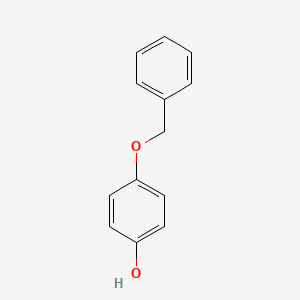 |
0.302 | ||
| ENC000676 | 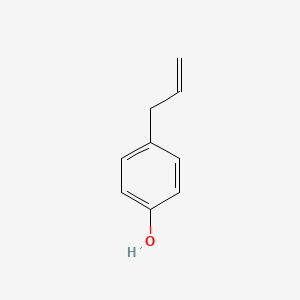 |
0.472 | D0V9EN |  |
0.277 | ||