NPs Basic Information
|
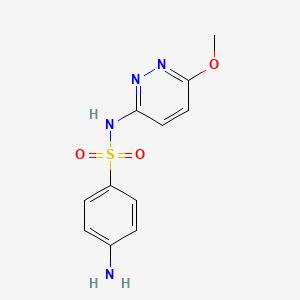
Name |
Sulfamethoxypyridazine
|
| Molecular Formula | C11H12N4O3S | |
| IUPAC Name* |
4-amino-N-(6-methoxypyridazin-3-yl)benzenesulfonamide
|
|
| SMILES |
COC1=NN=C(C=C1)NS(=O)(=O)C2=CC=C(C=C2)N
|
|
| InChI |
InChI=1S/C11H12N4O3S/c1-18-11-7-6-10(13-14-11)15-19(16,17)9-4-2-8(12)3-5-9/h2-7H,12H2,1H3,(H,13,15)
|
|
| InChIKey |
VLYWMPOKSSWJAL-UHFFFAOYSA-N
|
|
| Synonyms |
sulfamethoxypyridazine; 80-35-3; Sulphamethoxypyridazine; Midicel; Sulfapyridazine; Depovernil; Sulfalex; Sulfametoxipiridazine; Spofadazine; Sulfdurazin; Lederkyn; Longin; 3-Sulfa-6-methoxypyridazine; Sulfamethoxypyridazinum; Lisulfen; Petrisul; Piridolo; Quinoseptyl; Retasulfin; Sulfametoxipiridazina; Sulfozona; Sultirene; Altezol; Davosin; Lentac; Medicel; Midikel; Myasul; Opinsul; Paramid; Retamid; Slosul; Vinces; Durox; Kinex; Kynex; Smop; Solfametossipiridazina; Paramid Supra; 3-Methoxy-6-sulfanylamidopyridazine; 3-Sulfanilamide-6-methoxypyridazine; 3-Sulfanilamido-6-methoxypyridazine; 6-Methoxy-3-sulfanilamidopyridazine; 6-Sulfanilamido-3-methoxypyridazine; 4-amino-N-(6-methoxypyridazin-3-yl)benzenesulfonamide; Retasulphine; 3-(p-Aminobenzenesulfamido)-6-methoxypyridazine; 3-p-Aminobenzenesulphonamido-6-methoxypyridazine; Benzenesulfonamide, 4-amino-N-(6-methoxy-3-pyridazinyl)-; sulfamethoxipyridazine; 4-amino-N-(6-methoxy-3-pyridazinyl)benzenesulfonamide; 4-Amino-N-(6-methoxy-3-pyridazinyl)-benzenesulfonamide; CL 13494; 6-Methoxy-3-pyridazinylsulfanilamide; Sulfamethoxypyridazine [INN]; RP 7522; CHEBI:102516; 3-(4-Aminobenzenesulfonamido)-6-methoxypyridazine; N(sup 1)-(6-Methoxy-3-pyridazinyl)sulfanilamide; NSC-757875; MLS000069641; T034E4NS2Z; N(1)-(6-Methoxy-3-pyridazinyl)sulfanilamide; NCGC00016324-01; CL-13494; N-(6-Methoxy-3-pyridazinyl)sulfanilamide; SMR000018386; Sulfamethoxipyridazinum; DSSTox_CID_3611; DSSTox_RID_77108; DSSTox_GSID_23611; Sulfamethoxypyridazine (INN); Sulfamethoxypyridazine 100 microg/mL in Acetonitrile; CAS-80-35-3; Solfametossipiridazina [DCIT]; SR-01000000179; Sulfamethoxypyridazinum [INN-Latin]; Sulfametoxipiridazina [INN-Spanish]; EINECS 201-272-5; BRN 0277076; UNII-T034E4NS2Z; Kineks; N1-(6-Methoxy-3-pyridazinyl)sulfanilamide; Lederkyn (TN); Prestwick_118; 4-Amino-N-(6-methoxy-3-pyridazinyl)benzolsulfonamid; Sulfamethoxypyridazine [USP:INN:BAN]; Spectrum_001151; Opera_ID_698; Prestwick0_000724; Prestwick1_000724; Prestwick2_000724; Prestwick3_000724; Pyridazine, sulfamethoxy-; Spectrum2_001429; Spectrum3_001462; Spectrum4_000430; Spectrum5_001187; Epitope ID:122239; Sulphamethazine Sodium Salt; Oprea1_275757; SCHEMBL93617; BSPBio_000648; BSPBio_002983; KBioGR_000760; KBioSS_001631; 5-25-12-00424 (Beilstein Handbook Reference); MLS000100719; MLS001148433; DivK1c_000239; SPECTRUM1501156; SPBio_001538; SPBio_002587; BPBio1_000714; CHEMBL268869; Sulfanilamide, N(sup 1)-(6-methoxy-3-pyridazinyl)-; 4-Amino-N-(6-methoxy-pyridazin-3-yl)-benzenesulfonamide; benzenesulfonamide,4-amino-n-(6-methoxy-3-pyridazinyl)-; DTXSID5023611; HMS500L21; KBio1_000239; KBio2_001631; KBio2_004199; KBio2_006767; KBio3_002483; ZINC49141; NINDS_000239; HMS1570A10; HMS1921L17; HMS2092J05; HMS2097A10; HMS2234F17; HMS3652H21; HMS3714A10; Pharmakon1600-01501156; SULFAMETHOXYPYRIDAZINE [MI]; 3-Methoxy-6-sulfanilamidopyridazine; HY-B1387; Tox21_110373; CCG-38976; MFCD00057372; NSC757875; s4250; SULFAMETHOXYPYRIDAZINE [MART.]; AKOS000605846; Tox21_110373_1; CS-4821; DB13773; NSC 757875; SDCCGMLS-0003277.P003; SULFAMETHOXYPYRIDAZINE [WHO-DD]; SULFAMETHOXYPYRIDAZINE [WHO-IP]; IDI1_000239; NCGC00016324-02; NCGC00016324-03; NCGC00016324-04; NCGC00016324-05; NCGC00016324-06; NCGC00016324-08; NCGC00016324-09; NCGC00022232-03; NCGC00022232-04; AC-12005; AC-32613; BS-32820; SBI-0051666.P002; Sulfamethoxypyridazine, analytical standard; AB00052228; FT-0653623; S0591; SW196429-3; SULFAMETHOXYPYRIDAZINUM [WHO-IP LATIN]; D02439; D70409; Sulfanilamide, N1-(6-methoxy-3-pyridazinyl)-; AB00052228_12; A839894; Q7636177; SR-01000000179-3; SR-01000000179-4; W-104237; BRD-K00938507-001-05-3; BRD-K00938507-001-13-7; Sulfamethoxypyridazine 1000 microg/mL in Acetonitrile; 4-amino-N-[6-(methyloxy)pyridazin-3-yl]benzenesulfonamide; Sulfamethoxypyridazine, VETRANAL(TM), analytical standard; Sulfamethoxypyridazine, European Pharmacopoeia (EP) Reference Standard
|
|
| CAS | 80-35-3 | |
| PubChem CID | 5330 | |
| ChEMBL ID | CHEMBL268869 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 280.31 | ALogp: | 0.3 |
| HBD: | 2 | HBA: | 7 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 116.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 19 | QED Weighted: | 0.817 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.915 | MDCK Permeability: | 0.00001360 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.049 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.17 | Plasma Protein Binding (PPB): | 83.91% |
| Volume Distribution (VD): | 0.774 | Fu: | 13.51% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.029 | CYP1A2-substrate: | 0.366 |
| CYP2C19-inhibitor: | 0.261 | CYP2C19-substrate: | 0.171 |
| CYP2C9-inhibitor: | 0.472 | CYP2C9-substrate: | 0.788 |
| CYP2D6-inhibitor: | 0.167 | CYP2D6-substrate: | 0.717 |
| CYP3A4-inhibitor: | 0.358 | CYP3A4-substrate: | 0.127 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.272 | Half-life (T1/2): | 0.109 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.033 | Human Hepatotoxicity (H-HT): | 0.879 |
| Drug-inuced Liver Injury (DILI): | 0.99 | AMES Toxicity: | 0.026 |
| Rat Oral Acute Toxicity: | 0.018 | Maximum Recommended Daily Dose: | 0.022 |
| Skin Sensitization: | 0.039 | Carcinogencity: | 0.619 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.142 |
| Respiratory Toxicity: | 0.118 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000112 | 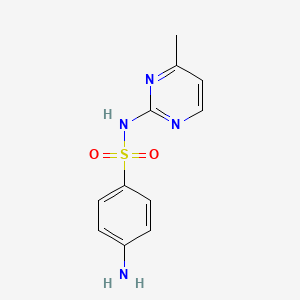 |
0.561 | D07SYJ | 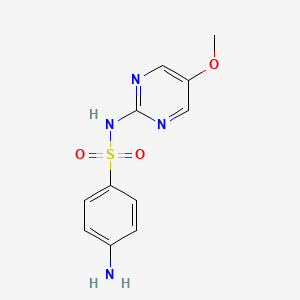 |
0.582 | ||
| ENC000109 | 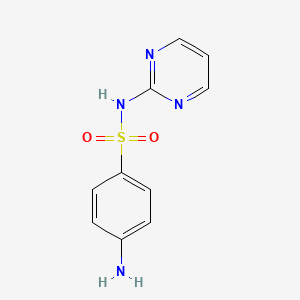 |
0.507 | D0R9OH | 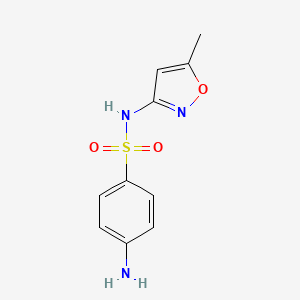 |
0.563 | ||
| ENC000113 | 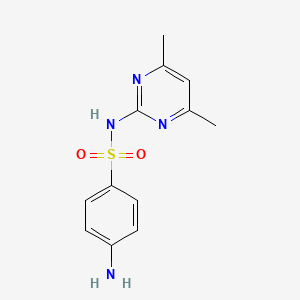 |
0.500 | D07JJS | 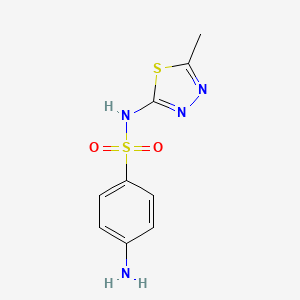 |
0.563 | ||
| ENC000111 | 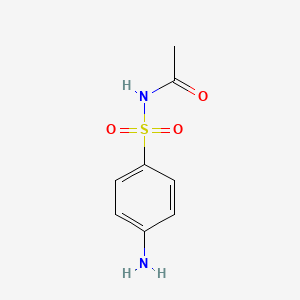 |
0.452 | D0H1GJ | 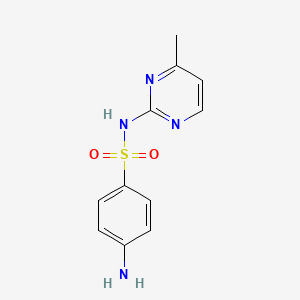 |
0.561 | ||
| ENC000115 | 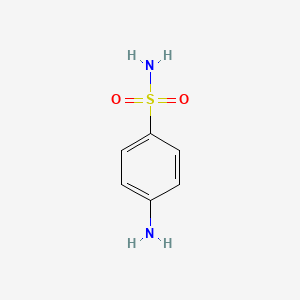 |
0.414 | D0D4CY | 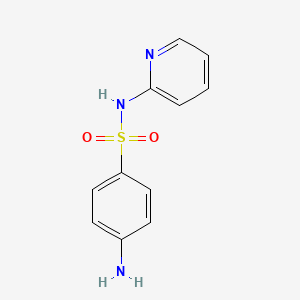 |
0.554 | ||
| ENC001224 | 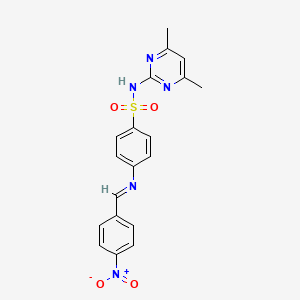 |
0.314 | D0T1GT | 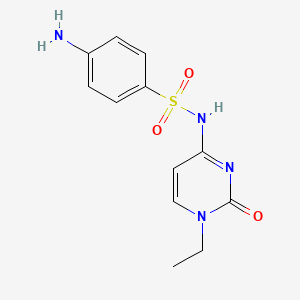 |
0.543 | ||
| ENC001517 | 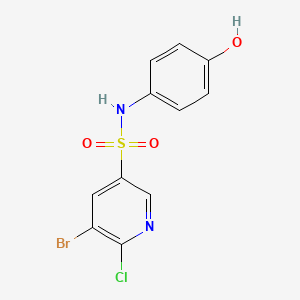 |
0.313 | D07PAO | 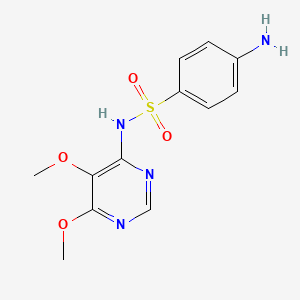 |
0.521 | ||
| ENC001351 | 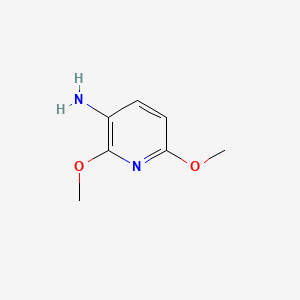 |
0.258 | D05LKP | 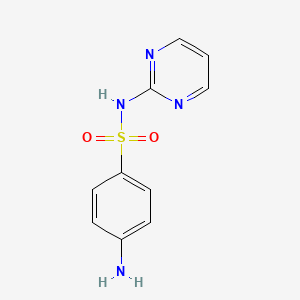 |
0.507 | ||
| ENC002077 | 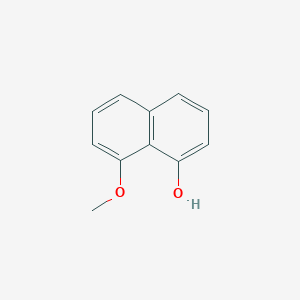 |
0.250 | D0V9YR | 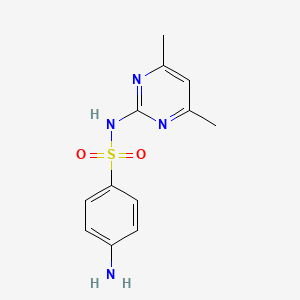 |
0.500 | ||
| ENC001512 | 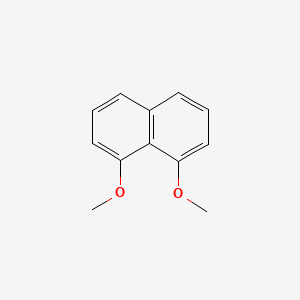 |
0.240 | D09TBD |  |
0.478 | ||