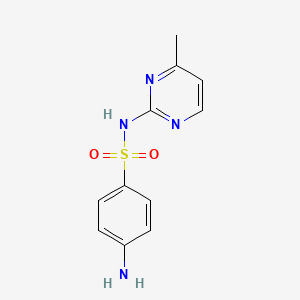
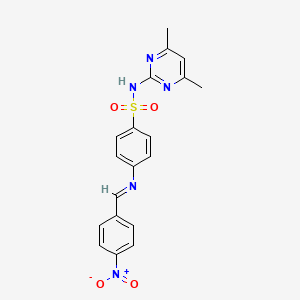
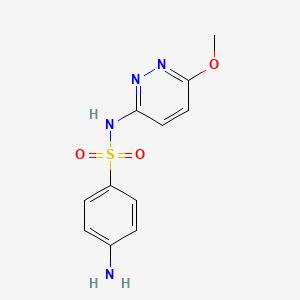
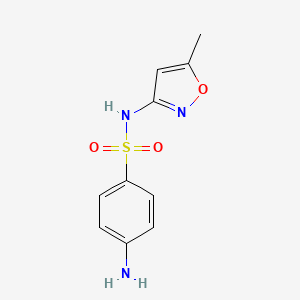
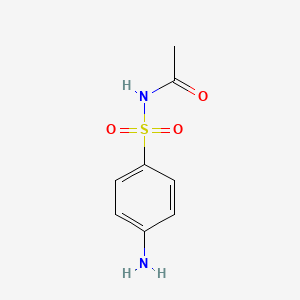
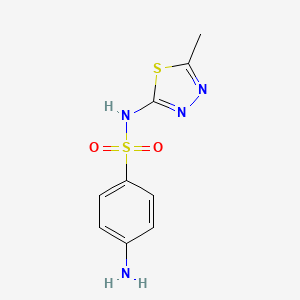
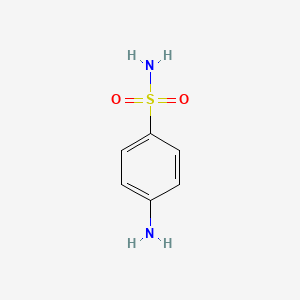
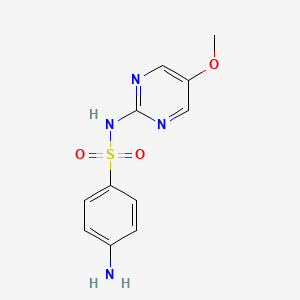
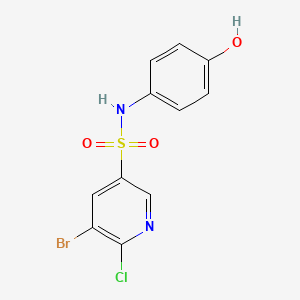
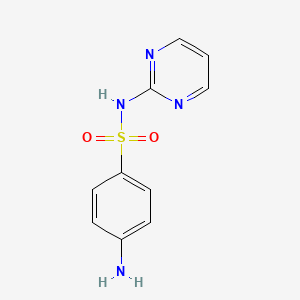
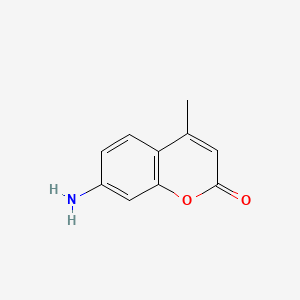
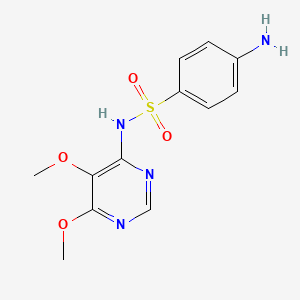
| Synonyms |
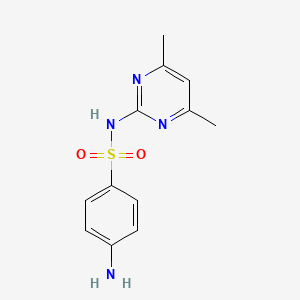
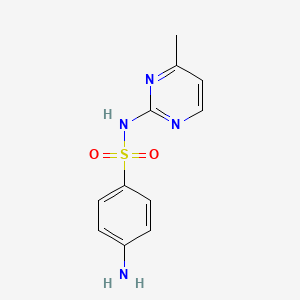
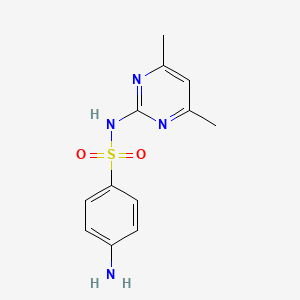
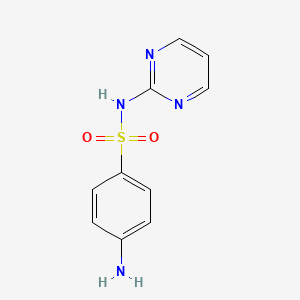
sulfamethazine; Sulfadimidine; 57-68-1; Sulfadimerazine; Sulfamezathine; Sulphamethazine; Sulfadimethyldiazine; Sulfadimethylpyrimidine; 4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide; Sulphamezathine; Sulfadimezine; Sulfadimidin; Sulphadimidine; Sulphadimethylpyrimidine; Sulfadimesin; Sulfadimesine; Sulfadimezin; Sulfadimidinum; Sulfametazyny; Sulfamethiazine; Sulphamethasine; Sulphamidine; Sulphodimezine; Sulfadine; Cremomethazine; Sulfadimidina; Sulfametazina; Sulfodimezine; Azolmetazin; Dimezathine; Intradine; Kelametazine; Pirmazin; Spanbolet; Sulfodimesin; Superseptil; Superseptyl; Vertolan; Diazil; Mermeth; Neasina; Neazina; Sulfa-Isodimerazine; Dimidin-R; Hava-Span; 4,6-Dimethyl-2-sulfanilamidopyrimidine; Calfspan Tablets; Sa III; 4-Amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide; Sulfamidine; 2-Sulfanilamido-4,6-dimethylpyrimidine; SulfaSURE SR Bolus; N-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; Benzenesulfonamide, 4-amino-N-(4,6-dimethyl-2-pyrimidinyl)-; Diazil (the sulfanilamide); Primazin; 2-(p-Aminobenzenesulfonamido)-4,6-dimethylpyrimidine; A-502; 6-(4'-Aminobenzol-sulfonamido)-2,4-dimethylpyrimidin; N(1)-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; NCI-C56600; N(1)-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; 4-Amino-N-(2,6-dimethyl-4-pyrimidinyl)benzenesulfonamide; Sulmet; Sulfadimidine;Sulfadimerazine; (p-Aminobenzolsulfonyl)-2-amino-4,6-dimethylpyrimidin; Sulfadimidine (INN); Sulfamethazine (USP); Sulfamethazine [USP]; 2-(4-Aminobenzenesulfonamido)-4,6-dimethylpyrimidine; N(sup 1)-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; CHEBI:102265; Sulfadimezinum; 4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzene-1-sulfonamide; NSC-67457; NSC-683529; Sulfanilamide, N(sup1)-(4,6-dimethyl-2-pyrimidinyl)-; MLS000069711; 4-Amino-N-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide; Solfadimidina; n(sup1)-(2,6-Dimethylpyrimid-4-yl)sulfanilamide; N(Sup1)-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; NSC67457; Sulfadimidine-d4; Sulfanilamide, N1-(4,6-dimethyl-2-pyrimidinyl)-; Sulka S Boluses; N(Sup1)-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; NSC683529; 48U51W007F; 4,6-Dimethylsulfadiazine; BN-2409; NCGC00018243-07; SMR000017409; Solfadimidina [DCIT]; Sulfametazyny [Polish]; DSSTox_CID_1290; Sulfamethazine 100 microg/mL in Acetonitrile; SULFADIMIDINE [INN]; DSSTox_RID_76062; DSSTox_GSID_21290; SMZ; Sulfadimidinum [INN-Latin]; Sulfadimidina [INN-Spanish]; Sulfametazina [Italian]; Sulfadimidine [INN:BAN]; CAS-57-68-1; BN 2409; CCRIS 3701; Sulfamezathine (TN); HSDB 4157; EINECS 200-346-4; MFCD00006066; NSC 67457; BRN 0261304; N(sup 1)-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; sulfamethazone; Diazilsulfadine; Calfspan; Panazin; AI3-26817; Sulka k boluses; S-Dimidine; Dimidim-R; UNII-48U51W007F; (p-Aminobenzolsulfonyl)-2-amino-4,6-dimethylpyrimidin [German]; 6-(4'-Aminobenzol-sulfonamido)-2,4-dimethylpyrimidin [German]; Sulfadimidine,(S); Sulfanilamide, N(1)-(4,6-dimethyl-2-pyrimidinyl)-; Sulfadimidine-13C6; 4-Amino-N-(4,6-dimethyl-2-pyrimidyl)benzenesulfonamide; Sentry aq mardel biospheres maracyn plus; Spectrum_000990; [(4-Aminophenyl)sulfonyl](4,6-dimethylpyrimidin-2-yl)amine; 4-amino-N-(4; Opera_ID_1374; Prestwick0_000775; Prestwick1_000775; Prestwick2_000775; Prestwick3_000775; Spectrum2_001321; Spectrum3_001700; Spectrum4_000344; Spectrum5_001270; Sulfamethazine, >=99%; CHEMBL446; Epitope ID:122238; Cambridge id 5251384; NCIOpen2_003489; BIDD:PXR0093; Oprea1_142608; Oprea1_677935; BSPBio_000850; BSPBio_003260; CBDivE_012932; KBioGR_000747; KBioSS_001470; SULFAMETHAZINE [HSDB]; SULFAMETHAZINE [IARC]; 5-25-10-00250 (Beilstein Handbook Reference); MLS000103403; MLS001077331; MLS002454449; DivK1c_000293; SCHEMBL151305; SPECTRUM1500548; SULFADIMIDINE [MART.]; SULFAMETHAZINE [VANDF]; SPBio_001441; SPBio_002789; SULFADIMIDINE [WHO-DD]; SULFADIMIDINE [WHO-IP]; BPBio1_000936; SULFAMETHAZINE [USP-RS]; DTXSID6021290; Sulfanilamide, N(sup 1)-(4,6-dimethyl-2-pyrimidinyl)-; ASWVTGNCAZCNNR-UHFFFAOYSA-; HMS500O15; KBio1_000293; KBio2_001470; KBio2_004038; KBio2_006606; KBio3_002480; ZINC57494; NINDS_000293; HMS1921A17; HMS2092I19; HMS3652K03; Pharmakon1600-01500548; SULFADIMIDINE [EP IMPURITY]; SULFAMETHAZINE [GREEN BOOK]; ALBB-033473; BCP28439; HY-B0035; SULFADIMIDINE [EP MONOGRAPH]; Sulfadimidine for peak identification; SULFAMETHAZINE [ORANGE BOOK]; Tox21_110847; Tox21_202221; Tox21_303006; CCG-39259; NSC757326; s3133; STK097514; SULFADIMIDINUM [WHO-IP LATIN]; SULFAMETHAZINE [USP MONOGRAPH]; AKOS000119894; SULFOSE COMPONENT SULFAMETHAZINE; Tox21_110847_1; DB01582; MS-1576; NSC-757326; IDI1_000293; TERFONYL COMPONENT SULFAMETHAZINE; LANTRISUL COMPONENT SULFAMETHAZINE; NCGC00018243-01; NCGC00018243-02; NCGC00018243-03; NCGC00018243-04; NCGC00018243-05; NCGC00018243-06; NCGC00018243-08; NCGC00018243-09; NCGC00021490-03; NCGC00021490-04; NCGC00021490-05; NCGC00021490-06; NCGC00256371-01; NCGC00259770-01; SULFALOID COMPONENT SULFAMETHAZINE; WLN: T6N CNJ BMSWR DZ& D1 F1; AC-16126; NEOTRIZINE COMPONENT SULFAMETHAZINE; SULFAMETHAZINE COMPONENT OF SULFOSE; Sulfanilamide,6-dimethyl-4-pyrimidinyl)-; SBI-0051522.P003; SULFAMETHAZINE (TRISULFAPYRIMIDINES); SULFAMETHAZINE COMPONENT OF TERFONYL; TRISULFAPYRIMIDINES (SULFAMETHAZINE); SULFAMETHAZINE COMPONENT OF LANTRISUL; SULFAMETHAZINE COMPONENT OF SULFALOID; FT-0655603; FT-0674743; N1-(4,6-Dimethyl-2-pyrimidyl)sulfanilamide; SULFAMETHAZINE COMPONENT OF NEOTRIZINE; SW219689-1; Benzenesulfonamide,6-dimethyl-4-pyrimidinyl)-; EN300-16843; TRIPLE SULFOID COMPONENT SULFAMETHAZINE; C19530; D02436; N1-(4,6-Dimethyl-2-pyrimidinyl)sulfanilamide; Sulfamethazine 1000 microg/mL in Acetonitrile; AB00052097_12; AB00052097_13; SULFAMETHAZINE COMPONENT OF TRIPLE SULFOID; A831551; SR-01000000211; Sulfamethazine, Vetec(TM) reagent grade, >=99%; Sulfamethazine, VETRANAL(TM), analytical standard; Q3976823; SR-01000000211-3; W-105450; 4-amino-N-(4,6-dimethyl-2-pyridyl)benzenesulfonamide; BRD-K11640013-001-02-6; BRD-K11640013-236-03-6; Z56791687; 2-(4-Aminobenzenesulfonylamino)-4,6-dimethylpyrimidine; F1443-4796; TRISULFAPYRIMIDINES (SULFAMETHAZINE) [ORANGE BOOK]; Sulfadimidine, European Pharmacopoeia (EP) Reference Standard; (4-AMINO-N-(4,6-DIMETHYL-2-PYRIMIDINYL)BENZENE SULFONAMIDE; 4-amino-N~1~-(4,6-dimethyl-2-pyrimidinyl)-1-benzenesulfonamide; HSDB 4157; HSDB 4157; HSDB 4157;Sulfadimidine;Sulfadimerazine; Sulfamethazine, United States Pharmacopeia (USP) Reference Standard; Sulfadimidine for peak identification, European Pharmacopoeia (EP) Reference Standard
|