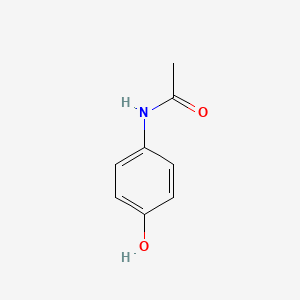
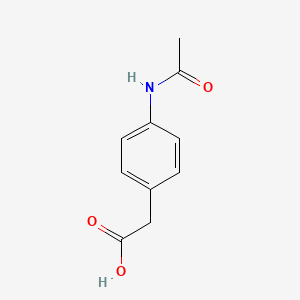
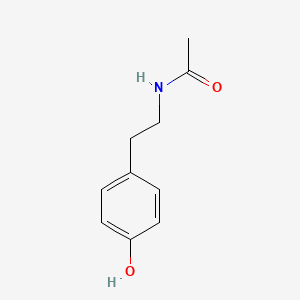
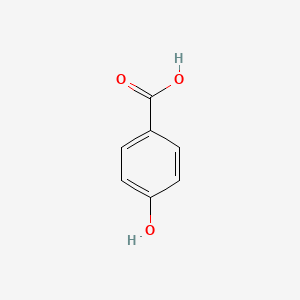
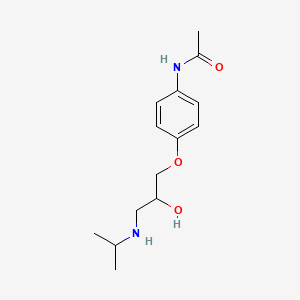
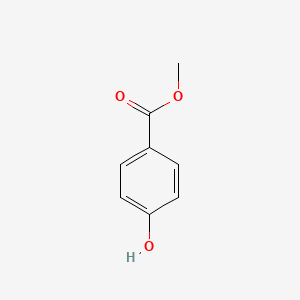
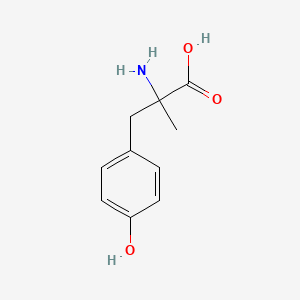
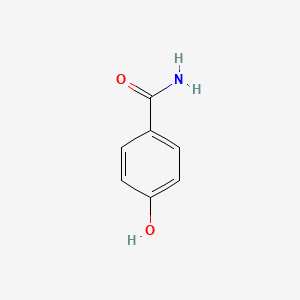
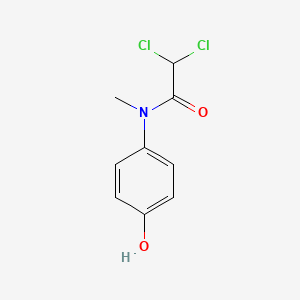
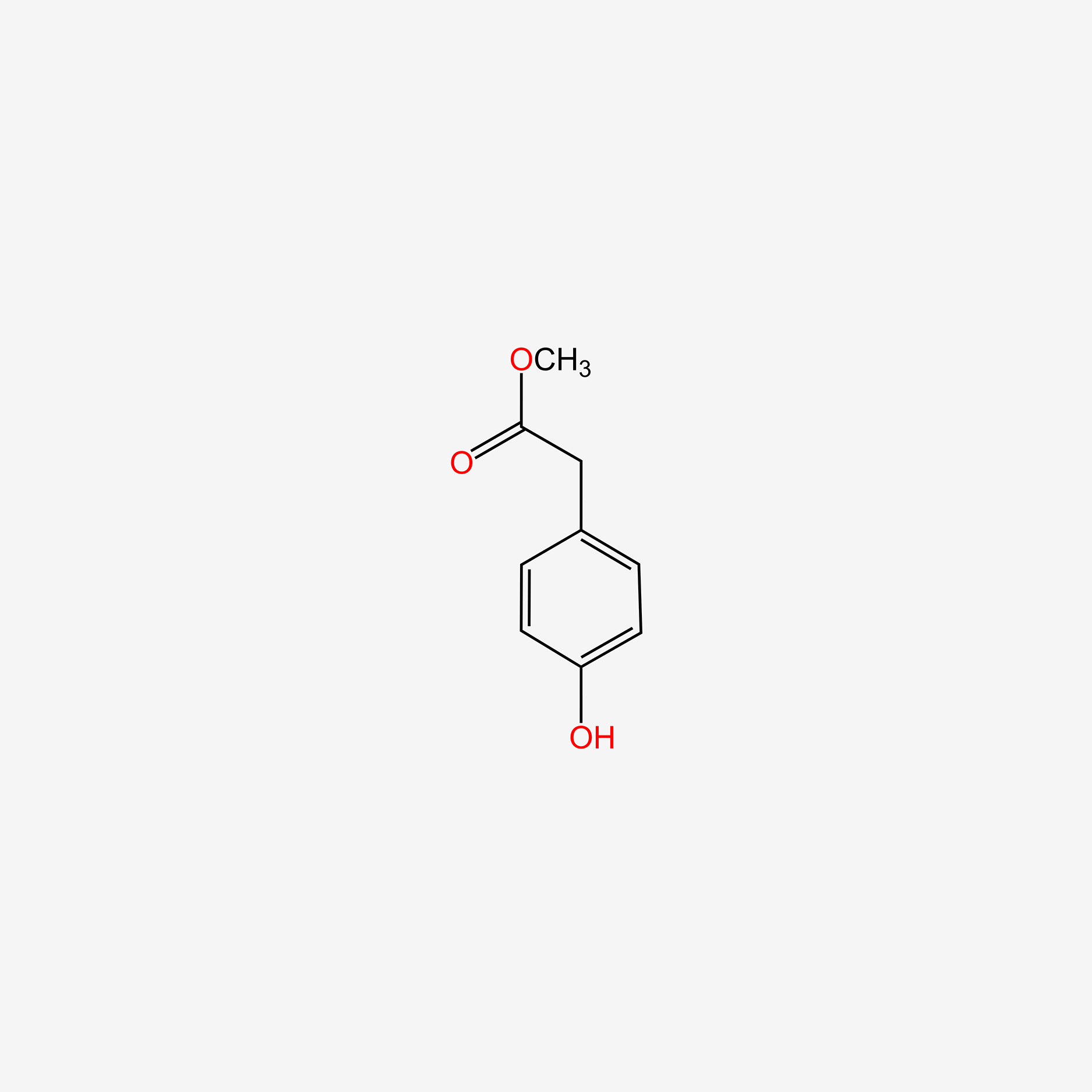
| InChI |
InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10)
|
| Synonyms |
acetaminophen; Paracetamol; 4-Acetamidophenol; 103-90-2; N-(4-Hydroxyphenyl)acetamide; Tylenol; APAP; Panadol; N-Acetyl-p-aminophenol; 4'-Hydroxyacetanilide; Acetaminofen; Datril; p-Hydroxyacetanilide; p-Acetamidophenol; Algotropyl; Lonarid; Naprinol; Doliprane; Injectapap; Acamol; Acenol; Anelix; Multin; p-Acetaminophenol; Abensanil; Acetagesic; Acetalgin; Biocetamol; Clixodyne; Gelocatil; Liquagesic; Pyrinazine; Servigesic; Acephen; Alvedon; Anaflon; Apamide; Dymadon; Febridol; Febrilix; Febrolin; Finimal; Homoolan; Lestemp; Paracet; Tabalgin; Tralgon; Tussapap; Valadol; Valgesic; Vermidon; Alpiny; Amadil; Anhiba; Calpol; Dirox; Eneril; Fendon; Hedex; Lyteca; Neopap; Pacemo; Panets; Parmol; Tapar; Tempra; Acetamide, N-(4-hydroxyphenyl)-; Paracetamolo; Dafalgan; Dolprone; Momentum; Ortensan; Paldesic; Banesin; Captin; Disprol; Enelfa; Salzone; Exdol; p-Acetylaminophenol; Febro-Gesic; NEBS; Paracetamolum; Dolgesic; Elixodyne; Febrectal; Phenaphen; Tempanal; Abenol; Apacet; Apadon; Cetadol; Fensum; Janupap; Minoset; Napafen; Neodol; Nobedon; Pacemol; Panodil; Parapan; Pedric; Phendon; Rounox; Suppap; Korum; Pinex; Temlo; 4-(Acetylamino)phenol; Ben-u-ron; Dial-A-Gesic; Anacin-3; Calmanticold; Codoliprane; Demogripal; Dolegrippin; Doloreduct; Dristancito; Duracetamol; Eu-Med; Grippostad; Gynospasmine; Medocodene; Paedialgon; Paracetanol; Parakapton; Pediapirin; Phenipirin; Phogoglandin; Predualito; Sanicopyrine; Scentalgyl; Sunetheton; Tachiprina; Termalgine; Treuphadol; Abrolet; Acertol; Acetamol; Acetofen; Afebrin; Afebryl; Aferadol; Algesidal; Algomol; Alpinyl; Analter; Antidol; Apitrelal; Atralidon; Babikan; Bacetamol; Cadafen; Calapol; Causalon; Cefalex; Codabrol; Codalgin; Codapane; Codicet; Codisal; Cofamol; Cosutone; Cuponol; Curadon; Custodial; Darocet; Daygrip; Deminofen; Democyl; Desfebre; Dimindol; Dolefin; Dolofugin; Dolorfug; Dolorstop; Dolotec; Dorocoff; Dularin; Durapan; Ecosetol; Excipain; Fanalgic; Farmadol; Febranine; Febrectol; Febricet; Febrinol; Fepanil; Finiweh; Fluparmol; Geluprane; Ildamol; Inalgex; Infadrops; Kataprin; Labamol; Lekadol; Lemgrip; Lupocet; Magnidol; Malidens; Maxadol; Mexalen; Minafen; Miralgin; Nealgyl; Neodolito; Neotrend; Neuridon; Nodolex; Ofirmev; Oralgan; Oxycocet; Pacimol; Panacete; Panadeine; Panadiene; Panaleve; Panamax; Panasorbe; Panofen; Pantalgin; Paracemol; Paracenol; Paracetol; Paracin; Paracod; Paracodol; Parador; Paradrops; Paralen; Paralief; Paralink; Paralyoc; Paramol; Paramolan; Paranox; Parasedol; Parasin; Paraspen; Parcetol; Parogal; Pediatrix; Perfalgan; Piramin; Pirinasol; Polmofen; Predimol; Prontina; Puernol; Pulmofen; Pyrigesic; Pyromed; Remedol; Rivalgyl; Rubophen; Rupemol; Sanicet; Schmerzex; Sedalito; Semolacin; Seskamol; Setakop; Setamol; Sifenol; Sinaspril; Sinedol; Stanback; Stopain; Supofen; Tazamol; Termacet; Termalgin; Termofren; Titralgan; Tricoton; Upsanol; Utragin; Veralgina; Viruflu; Vivimed; Zatinol; Abrol; Algina; Anapap; Andox; Asetam; Asomal; Aspac; Asplin; Benmyo; Curpol; Dhamol; Dolcor; Dolko; Dresan; Dypap; Febrex; Febrin; Lemsip; Malgis; Oltyl; Paceco; Pacet; Paedol; Painex; Pamol; Panex; Parake; Paroma; Plicet; Prodol; Reliv; Scanol; Setol; Sinmol; Tiffy; Tylex; Tylol; Tymol; Verpol; Volpan; Zolben; NeoCitran; NilnOcen; Nina; RubieMol; Vips; Supadol mono; Treupel mon; Bickie-mol; Fortalidon P; Gattaphen T; Gripin Bebe; Influbene N; Lonarid Mono; Panadeine Co; Dymadon Co; Toximer P; Treupel N; Accu-Tap; 4-Acetaminophenol; Helon N; Malex N; Spalt N; Tylex CD; N-Acetyl-4-aminophenol; SK-Apap; Paracetamole; Conacetol; Darvocet; Empracet; Panasorb; A-Per; Apamid; Parelan; Prompt; Vicodin; Fevor; Freka-cetamol; Codisal Forte; Croix Blanche; Dolorol Forte; Dymadon Forte; Junior Disprol; Kinder Finimal; Liquigesic Co; Mono Praecimed; Percocet-Demi; Perdolan Mono; Rockamol Plus; Viclor Richet; Actifed Plus; Kratofin simplex; Neo-Fepramol; Paracetamol AL; Paracetamol BC; Paracetamol PB; Acetanilide, 4'-hydroxy-; Claradol Codeine; Geralgine-P; Melabon Infantil; Migraleve Yellow; Paracetamol Saar; Pyregesic-C; Anti-Algos; Para-Suppo; Pasolind N; Supramol-M; No-Febril; Panado-Co; Para-Tabs; Paracetamol Hexal; Paracetamol Raffo; Paracetamol Rosch; Paracetamol Stada; Dol-Stop; Anadin dla dzieci; p-Hydroxyphenolacetamide; Percocet-5; Cod-Acamol Forte; Contra-Schmerz P; Hy-Phen; Medinol Paediatric; Paracetamol Basics; Panado-Co Caplets; Paracetamol von ct; Pe-Tam; Paracetamol Fecofar; Paracetamol Harkley; Paracetamol Heumann; Paracetamol Nycomed; Codral Pain Relief; Paracetamol Hanseler; Paracetamol Winthrop; 4-Hydroxyacetanilide; Phenaphen W/Codeine; Spalt fur die nacht; A.F. Anacin; Capital with Codeine; Paracetamol Genericon; Anexsia; Demilets; Efferalgan; Endecon; Intensin; Naldegesic; Propacet; Resfenol; Theraflu; Wygesic; Paracetamol Ratiopharm; Coricidin Sinus; Paracetamol Italfarmaco; Sudafed Sinus; Coricidin D; Paracetamol DC; Quiet World; Paracetamol Antipanin P; St Joseph Aspirin-Free; New Cortal for Children; INFANTS' FEVERALL; St. Joseph Fever Reducer; Midol Teen Formula; Paracetamol Dr. Schmidgall; Acetamide, N-(p-hydroxyphenyl)-; p-hydroxy-acetanilid; Aspirin-Free Anacin; Children's Tylenol Chewable; NCI-C55801; PCM Paracetamol Lichtenstein; Tylenol Allergy Sinus; p-(Acetylamino)phenol; Rhinex D-Lay Tablets; acetaminophene; Midol Regular Strength; Paracetamol SmithKline Beecham; Scherzatabletten Rezeptur 534; Percogesic with Codeine; 4-Hydroxyanilid kyseliny octove; Bayer Select Head Cold; Bayer Select Allergy-Sinus; Bayer Select Headache Pain; Dristan Cold No Drowsiness; Prestwick_13; St Joseph Aspirin-Free for Children; 4-acetylaminophenol; Children's Acetaminophen Elixir Drops; MFCD00002328; Midol PM Night Time Formula; Triaminic Sore Throat Formula; N-(4-hydroxyphenyl)ethanamide; Bayer Select Sinus Pain Relief; Phenol, p-acetamido-; Sine-Off Sinus Medicine Caplets; CHEBI:46195; Roxicet 5/500; Tocris-1706; NSC-3991; N-acetyl-para-aminophenol; 4-(N-Acetylamino)phenol; Acetaminophen (4-hydroxyacetanilide); Bayer Select Menstrual Multi-Symptom; Acetaco; n-acetyl-4-hydroxyaniline; St. Joseph Cold Tablets for Children; CHEMBL112; NSC-109028; N-(4-Hydroxyphenyl)acetamide (Tylenol); N-(4-hydroxyphenyl)-acetamide; 362O9ITL9D; Aminofen; DTXSID2020006; Atasol; Duaneo; Duorol; component of Dialog; component of Dilone; Fever All; Paracetamol (INN); component of Endecon; component of Percocet; component of Phenaphen; TYL; component of Percogesic; DSSTox_CID_6; NCGC00016361-07; Acetominophen; Actamin; CAS-103-90-2; Pasolind; Redutemp; Robigesic; Valorin; Aceta Elixir; PARACETAMOL [INN]; Dafalgan Codeine; Jin Gang; WLN: QR DMV1; DSSTox_RID_75318; DSSTox_GSID_20006; component of Hycomine Compound; Acetavance; Paracetamolo [Italian]; Calonal; Flexsure; Acenol (pharmaceutical); N-(4-Hydroxyphenyl)acetanilide; Drixoral Sinus; Aceta Tablets; Paracetamol [INN:BAN]; Valorin Extra; CCRIS 3; Snaplets-FR; Oraphen-PD; Phenaphen Caplets; Paracetamolum [INN-Latin]; Tylenol (caplet); Tylenol (geltab); Tylenol 8-Hour; SMR000112065; Tavist Allergy/Sinus/Headache; Dapa X-S; Drixoral Cold & Flu; HSDB 3001; SR-01000597517; EINECS 203-157-5; 222 AF; Acetaminophen [USP:JAN]; paracetamol (acetaminophen); NSC 109028; acetominophene; UNII-362O9ITL9D; 4-Hydroxyanilid kyseliny octove [Czech]; Claratal; Daphalgan; Resprin; Apacet Capsules; Atasol Caplets; Atasol Tablets; Tempra Caplets; Tylenol Caplets; Tylenol Elixir; Tylenol Gelcaps; Tylenol Tablets; Actamin Extra; Actamin Super; Aminofen Max; ANEXSIA 10/660; Apacet Elixir; Atasol Drops; Exdol Strong; p-Acetoaminophen; Tempra Drops; Tylenol Drops; alpha-Per; Citramon P; Excedrin Caplets; Dial-alpha-gesic; Apo-Acetaminophen; 4-acetominophenol; Genebs X-Tra; Paracetamol;Tylenol; 4-acetamido phenol; 4-acetamido-phenol; Tempra D.S; APAP, Paracetamol; p-hydroxyacetoanilide; Tylenol (TN); Supac (Salt/Mix); Tylox (Salt/Mix); Zydone (Salt/Mix); Atasol Forte Caplets; Atasol Forte Tablets; para-acetylaminophenol; Anexsia (Salt/Mix); Endecon (Salt/Mix); Sinubid (Salt/Mix); Talacen (Salt/Mix); Vicodin (Salt/Mix); Wygesic (Salt/Mix); Acetaminophen Uniserts; Datril Extra-Strength; Tylenol Infants Drops; Demilets (Salt/Mix); Empracet (Salt/Mix); Intensin (Salt/Mix); Propacet (Salt/Mix); Suppap-120; Suppap-325; Suppap-650; Panadol Extra Strength; TheraFlu (Salt/Mix); Coricidin (Salt/Mix); Liquiprin (Salt/Mix); Hy-Phen (Salt/Mix); IV-APAP; phenol derivative, 11; Spectrum_000016; Tempra Chewable Tablets; Naldegesic (Salt/Mix); Actimol Chewable Tablets; Feverall Junior Strength; Darvocet-N (Salt/Mix); Anacin-3 Extra Strength; Liquiprin Infants" Drops; N-acetyl para aminophenol; Prestwick0_000868; Prestwick1_000868; Prestwick2_000868; Prestwick3_000868; Spectrum2_000085; Spectrum3_000283; Spectrum4_000140; Spectrum5_000736; Coricidin D (Salt/Mix); Quiet World (Salt/Mix); Genapap Children's Elixir; Tylenol Children's Elixir; 4-Acetamidophenol, 98%; Actifed Plus (Salt/Mix); ACETAMINOPHEN [MI]; PARACETAMOL [IARC]; Epitope ID:117710; Genapap Children's Tablets; Sudafed Sinus (Salt/Mix); ACETAMINOPHEN [JAN]; EC 203-157-5; Actimol Infants' Suspension; Drixoral Sinus (Salt/Mix); Liquiprin Children's Elixir; SCHEMBL3480; Acetaminophen (JP17/USP); ACETAMINOPHEN [HSDB]; ACETAMINOPHEN [INCI]; Coricidin Sinus (Salt/Mix); N-(4-hydroxyfenyl)ethanamid; PARACETAMOL [MART.]; ACETAMINOPHEN [VANDF]; BSPBio_000915; BSPBio_001786; DDS-06A; KBioGR_000560; KBioSS_000356; PARACETAMOL [WHO-DD]; PARACETAMOL [WHO-IP]; 4-13-00-01091 (Beilstein Handbook Reference); Actimol Children's Suspension; Apacet Extra Strength Caplets; Apacet Extra Strength Tablets; Aspirin-Free Excedrin Caplets; Genebs Extra Strength Caplets; MLS001146925; MLS001331684; MLS002154041; BIDD:GT0005; DivK1c_000660; SPECTRUM1500101; Genapap Extra Strength Caplets; Genapap Extra Strength Tablets; SPBio_000010; SPBio_002836; Tapanol Extra Strength Caplets; Tapanol Extra Strength Tablets; Tylenol Extra Strength Caplets; Tylenol Extra Strength Gelcaps; Tylenol Extra Strength Tablets; ACETAMINOPHEN [USP-RS]; Actimol Junior Strength Caplets; Apacet Regular Strength Tablets; BPBio1_001007; Excedrin Extra Strength Caplets; Genebs Regular Strength Tablets; GTPL5239; Panadol Junior Strength Caplets; SGCUT00014; Tylenol Junior Strength Caplets; Midol Teen Formula (Salt/Mix); Genapap Regular Strength Tablets; Panadol Maximum Strength Caplets; Panadol Maximum Strength Tablets; SCHEMBL19474893; Tylenol Regular Strength Caplets; Tylenol Regular Strength Tablets; Aspirin-Free Anacin (Salt/Mix); BDBM26197; HMS502A22; KBio1_000660; KBio2_000356; KBio2_002924; KBio2_005492; KBio3_001286; NSC3991; Tylenol Arthritis Extended Relief; Acetaminophen, analytical standard; NINDS_000660; Tylenol Infants" Suspension Drops; BCPP000441; Drixoral Cold & Flu (Salt/Mix); HMS1570N17; HMS1920A03; HMS2091G03; HMS2097N17; HMS2269G20; HMS3268A10; HMS3412D16; HMS3676D16; HMS3714N17; PARACETAMOL [EP MONOGRAPH]; Pharmakon1600-01500101; Tylenol Allergy Sinus (Salt/Mix); ACETAMINOPHEN [ORANGE BOOK]; Midol Regular Strength (Salt/Mix); ACT06727; ALLAY COMPONENT ACETAMINOPHEN; AMY39958; BCP23431; BUCET COMPONENT ACETAMINOPHEN; ESGIC COMPONENT ACETAMINOPHEN; NORCO COMPONENT ACETAMINOPHEN; NSC 3991; STR00901; to_000023; TRIAD COMPONENT ACETAMINOPHEN; Tylenol Children's Chewable Tablets; TYLOX COMPONENT ACETAMINOPHEN; ACETAMINOPHEN [USP IMPURITY]; Acetaminophen, BioXtra, >=99.0%; Bayer Select Head Cold (Salt/Mix); Robitussin Night Relief (Salt/Mix); Tox21_110397; Tox21_201930; Tox21_300100; AC8790; ACETAMINOPHEN [USP MONOGRAPH]; BANCAP COMPONENT ACETAMINOPHEN; BBL005229; CCG-38901; CODRIX COMPONENT ACETAMINOPHEN; FEMCET COMPONENT ACETAMINOPHEN; LORTAB COMPONENT ACETAMINOPHEN; NORCET COMPONENT ACETAMINOPHEN; NSC109028; NSC755853; OXYCET COMPONENT ACETAMINOPHEN; PARACETAMOLUM [WHO-IP LATIN]; STL140694; TENCON COMPONENT ACETAMINOPHEN; TREZIX COMPONENT ACETAMINOPHEN; Tylenol Children's Suspension Liquid; ZINC13550868; ZYDONE COMPONENT ACETAMINOPHEN; ANEXSIA COMPONENT ACETAMINOPHEN; ANOQUAN COMPONENT ACETAMINOPHEN; BUTAPAP COMPONENT ACETAMINOPHEN; ROXICET COMPONENT ACETAMINOPHEN; ROXILOX COMPONENT ACETAMINOPHEN; SEDAPAP COMPONENT ACETAMINOPHEN; TALACEN COMPONENT ACETAMINOPHEN; TYCOLET COMPONENT ACETAMINOPHEN; VICODIN COMPONENT ACETAMINOPHEN; WYGESIC COMPONENT ACETAMINOPHEN; AKOS000121004; DARVOCET COMPONENT ACETAMINOPHEN; EXCEDRIN COMPONENT ACETAMINOPHEN; Feverall Sprinkle Caps Junior Strength; FIORICET COMPONENT ACETAMINOPHEN; HY-PHEN COMPONENT ACETAMINOPHEN; Ornex Severe Cold Formula (Salt/Mix); PERCOCET COMPONENT ACETAMINOPHEN; Tox21_110397_1; TRIAPRIN COMPONENT ACETAMINOPHEN; ULTRACET COMPONENT ACETAMINOPHEN; XARTEMIS COMPONENT ACETAMINOPHEN; ACETAMINOPHEN COMPONENT OF ALLAY; ACETAMINOPHEN COMPONENT OF BUCET; ACETAMINOPHEN COMPONENT OF ESGIC; ACETAMINOPHEN COMPONENT OF NORCO; ACETAMINOPHEN COMPONENT OF TRIAD; ACETAMINOPHEN COMPONENT OF TYLOX; Bayer Select Allergy-Sinus (Salt/Mix); BCP9000225; CO-GESIC COMPONENT ACETAMINOPHEN; DB00316; DHC PLUS COMPONENT ACETAMINOPHEN; NSC-755853; ACETAMINOPHEN COMPONENT OF BANCAP; ACETAMINOPHEN COMPONENT OF CODRIX; ACETAMINOPHEN COMPONENT OF FEMCET; ACETAMINOPHEN COMPONENT OF LORTAB; ACETAMINOPHEN COMPONENT OF NORCET; ACETAMINOPHEN COMPONENT OF OXYCET; ACETAMINOPHEN COMPONENT OF TENCON; ACETAMINOPHEN COMPONENT OF ZYDONE; BANCAP HC COMPONENT ACETAMINOPHEN; IDI1_000660; LORCET-HD COMPONENT ACETAMINOPHEN; PHRENILIN COMPONENT ACETAMINOPHEN; Sine-Aid, Maximum Strength (Salt/Mix); Sudafed Severe Cold Formula (Salt/Mix); ACETAMINOPHEN COMPONENT OF ANEXSIA; ACETAMINOPHEN COMPONENT OF ANOQUAN; ACETAMINOPHEN COMPONENT OF BUTAPAP; ACETAMINOPHEN COMPONENT OF ROXICET; ACETAMINOPHEN COMPONENT OF ROXILOX; ACETAMINOPHEN COMPONENT OF SEDAPAP; ACETAMINOPHEN COMPONENT OF TALACEN; ACETAMINOPHEN COMPONENT OF TYCOLET; ACETAMINOPHEN COMPONENT OF VICODIN; ACETAMINOPHEN COMPONENT OF WYGESIC; NCGC00016361-01; NCGC00016361-02; NCGC00016361-03; NCGC00016361-04; NCGC00016361-05; NCGC00016361-06; NCGC00016361-08; NCGC00016361-09; NCGC00016361-10; NCGC00016361-12; NCGC00016361-13; NCGC00016361-20; NCGC00025267-01; NCGC00025267-02; NCGC00025267-03; NCGC00025267-04; NCGC00025267-05; NCGC00253912-01; NCGC00259479-01; Tylenol Junior Strength Chewable Tablets; 8055-08-1; AC-23969; ACETAMINOPHEN COMPONENT OF DARVOCET; ACETAMINOPHEN COMPONENT OF EXCEDRIN; ACETAMINOPHEN COMPONENT OF FIORICET; ACETAMINOPHEN COMPONENT OF HY-PHEN; ACETAMINOPHEN COMPONENT OF PERCOCET; ACETAMINOPHEN COMPONENT OF TRIAPRIN; ACETAMINOPHEN COMPONENT OF ULTRACET; ACETAMINOPHEN COMPONENT OF XARTEMIS; HY-66005; Midol PM Night Time Formula (Salt/Mix); SY001162; Triaminic Sore Throat Formula (Salt/Mix); ACETAMINOPHEN COMPONENT OF CO-GESIC; ACETAMINOPHEN COMPONENT OF DHC PLUS; ACETAMINOPHEN COMPONENT OF PHRENILIN; BCP0726000305; DURADYNE DHC COMPONENT ACETAMINOPHEN; SBI-0051269.P003; ACETAMINOPHEN COMPONENT OF BANCAP HC; ACETAMINOPHEN COMPONENT OF LORCET-HD; Bayer Select Sinus Pain Relief (Salt/Mix); DRIXORAL PLUS COMPONENT ACETAMINOPHEN; AB00051905; Aspirin Free Anacin Maximum Strength Caplets; Aspirin Free Anacin Maximum Strength Tablets; DOLENE AP-65 COMPONENT ACETAMINOPHEN; FT-0658035; FT-0661041; FT-0661042; H0190; MEDIGESIC PLUS COMPONENT ACETAMINOPHEN; Sine-Off Sinus Medicine Caplets (Salt/Mix); SYNALGOS-DC-A COMPONENT ACETAMINOPHEN; ACETAMINOPHEN COMPONENT OF DURADYNE DHC; EN300-17510; N-(4-Hydroxyphenyl)-acetamide-[13C2,15N]; ACETAMINOPHEN COMPONENT OF DOLENE AP-65; ACETAMINOPHEN COMPONENT OF DRIXORAL PLUS; ACETAMINOPHEN COMPONENT OF SYNALGOS-DC-A; C06804; D00217; Q57055; AB00051905-09; AB00051905_10; ACETAMINOPHEN COMPONENT OF MEDIGESIC PLUS; Aspirin Free Anacin Maximum Strength Gel Caplets; Bayer Select Menstrual Multi-Symptom (Salt/Mix); Contac Cough & Sore Throat Formula (Salt/Mix); L024125; St. Joseph Cold Tablets for Children (Salt/Mix); Tylenol Extra Strength Adult Liquid Pain Reliever; J-001064; J-514275; Paracetamol (Acetaminophen) 1.0 mg/ml in Methanol; SR-01000597517-1; SR-01000597517-2; SR-01000597517-4; Acetaminophenol 4-Acetamino phenol Paracetamol Somedon; BRD-K41524689-001-08-6; Z56946051; F3096-1731; F48B493F-B1FD-410C-AA0A-F40EC71A0689; Bayer Select Maximum Strength Headache Pain Relief Formula; Paracetamol, British Pharmacopoeia (BP) Reference Standard; Paracetamol, European Pharmacopoeia (EP) Reference Standard; Acetaminophen, United States Pharmacopeia (USP) Reference Standard; Acetaminophen, meets USP testing specifications, 98.0-102.0%, powder; Acetaminophen, Pharmaceutical Secondary Standard; Certified Reference Material; Paracetamol for equipment qualification, EuropePharmacopoeia (EP) Reference Standard
|