NPs Basic Information
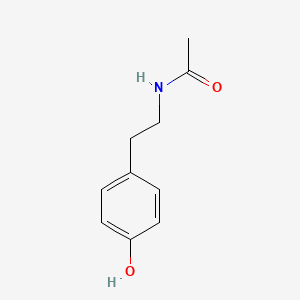
|
Name |
N-Acetyltyramine
|
| Molecular Formula | C10H13NO2 | |
| IUPAC Name* |
N-[2-(4-hydroxyphenyl)ethyl]acetamide
|
|
| SMILES |
CC(=O)NCCC1=CC=C(C=C1)O
|
|
| InChI |
InChI=1S/C10H13NO2/c1-8(12)11-7-6-9-2-4-10(13)5-3-9/h2-5,13H,6-7H2,1H3,(H,11,12)
|
|
| InChIKey |
ATDWJOOPFDQZNK-UHFFFAOYSA-N
|
|
| Synonyms |
N-Acetyltyramine; 1202-66-0; N-(4-Hydroxyphenethyl)acetamide; N-[2-(4-Hydroxyphenyl)ethyl]acetamide; N-Acetyl tyramine; N-(p-Hydroxyphenethyl)acetamide; N-(2-(4-Hydroxyphenyl)ethyl)acetamide; GNF-PF-5230; Acetyltyramine, N-; Acetamide, N-[2-(4-hydroxyphenyl)ethyl]-; MFCD01670887; BZB50E9QVY; CHEMBL152117; Acetamide, N-(2-(4-hydroxyphenyl)ethyl)-; Acetamide, N-(p-hydroxyphenethyl)-; UNII-BZB50E9QVY; BRN 2096467; N-Acetyl-tyramine; TYRAMINE, N-ACETYL; 4-13-00-01794 (Beilstein Handbook Reference); MLS000877027; SCHEMBL734658; 4-(2-acetamino-ethyl)-phenol; MEGxm0_000183; ACon1_000453; AMY5135; DTXSID60152731; N-(p-Hydroxyphenethyl) acetamide; CHEBI:125610; N-(4-hydroxyphenylethyl)acetamide; HMS2269F23; BAA20266; ZINC5160274; BDBM50136842; ZB0388; AKOS010245572; N-[2-(4-hydroxyphenyl)ethylacetamide; DS-3568; N-(4-Hydroxyphenethyl)acetamide, 95%; NCGC00169064-01; SMR000440653; SY103177; N-[2-(4-Hydroxyphenyl)ethyl]acetamide #; HY-120504; N-[2-(4-Hydroxy-phenyl)-ethyl]-acetamide; N-Acetyltyramine, >=95% (LC/MS-ELSD); CS-0078197; FT-0693682; Y10620; 202A660; A892285; BRD-K65981456-001-01-5; Q27216226; NCGC00169064-02!N-[2-(4-hydroxyphenyl)ethyl]acetamide
|
|
| CAS | 1202-66-0 | |
| PubChem CID | 121051 | |
| ChEMBL ID | CHEMBL152117 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 179.22 | ALogp: | 1.1 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 49.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.739 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.451 | MDCK Permeability: | 0.00001210 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.473 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.688 |
| 30% Bioavailability (F30%): | 0.975 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.626 | Plasma Protein Binding (PPB): | 22.34% |
| Volume Distribution (VD): | 1.063 | Fu: | 65.80% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.424 | CYP1A2-substrate: | 0.403 |
| CYP2C19-inhibitor: | 0.279 | CYP2C19-substrate: | 0.356 |
| CYP2C9-inhibitor: | 0.068 | CYP2C9-substrate: | 0.659 |
| CYP2D6-inhibitor: | 0.327 | CYP2D6-substrate: | 0.718 |
| CYP3A4-inhibitor: | 0.03 | CYP3A4-substrate: | 0.218 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.964 | Half-life (T1/2): | 0.873 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.037 | Human Hepatotoxicity (H-HT): | 0.207 |
| Drug-inuced Liver Injury (DILI): | 0.092 | AMES Toxicity: | 0.143 |
| Rat Oral Acute Toxicity: | 0.051 | Maximum Recommended Daily Dose: | 0.136 |
| Skin Sensitization: | 0.231 | Carcinogencity: | 0.347 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.143 |
| Respiratory Toxicity: | 0.022 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005495 | 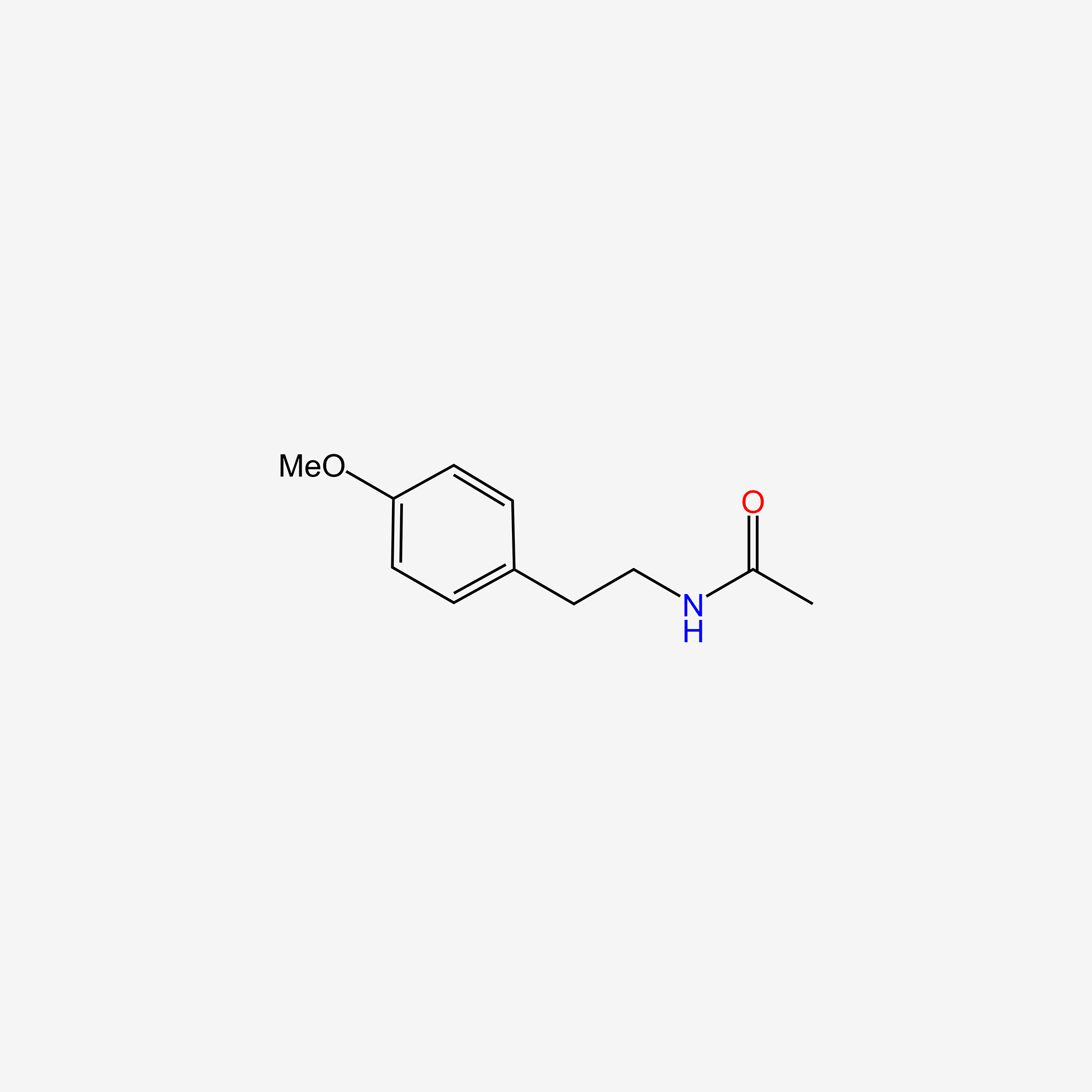 |
0.667 | D0U5QK |  |
0.535 | ||
| ENC001422 |  |
0.636 | D0B3QM | 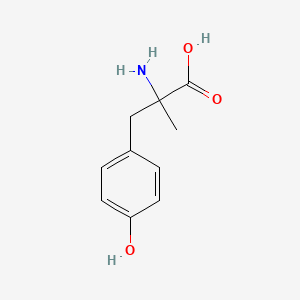 |
0.490 | ||
| ENC000350 | 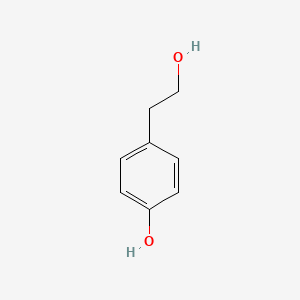 |
0.561 | D01CRB | 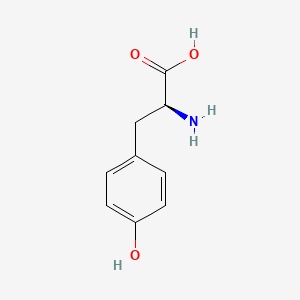 |
0.479 | ||
| ENC000693 | 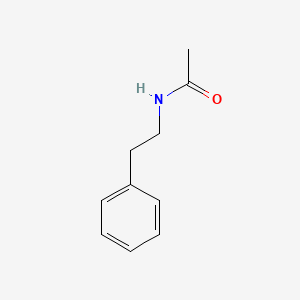 |
0.556 | D0W1RY | 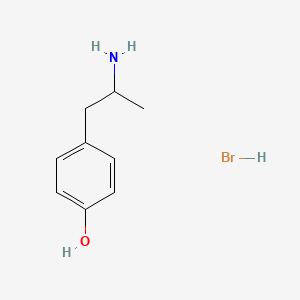 |
0.457 | ||
| ENC005812 | 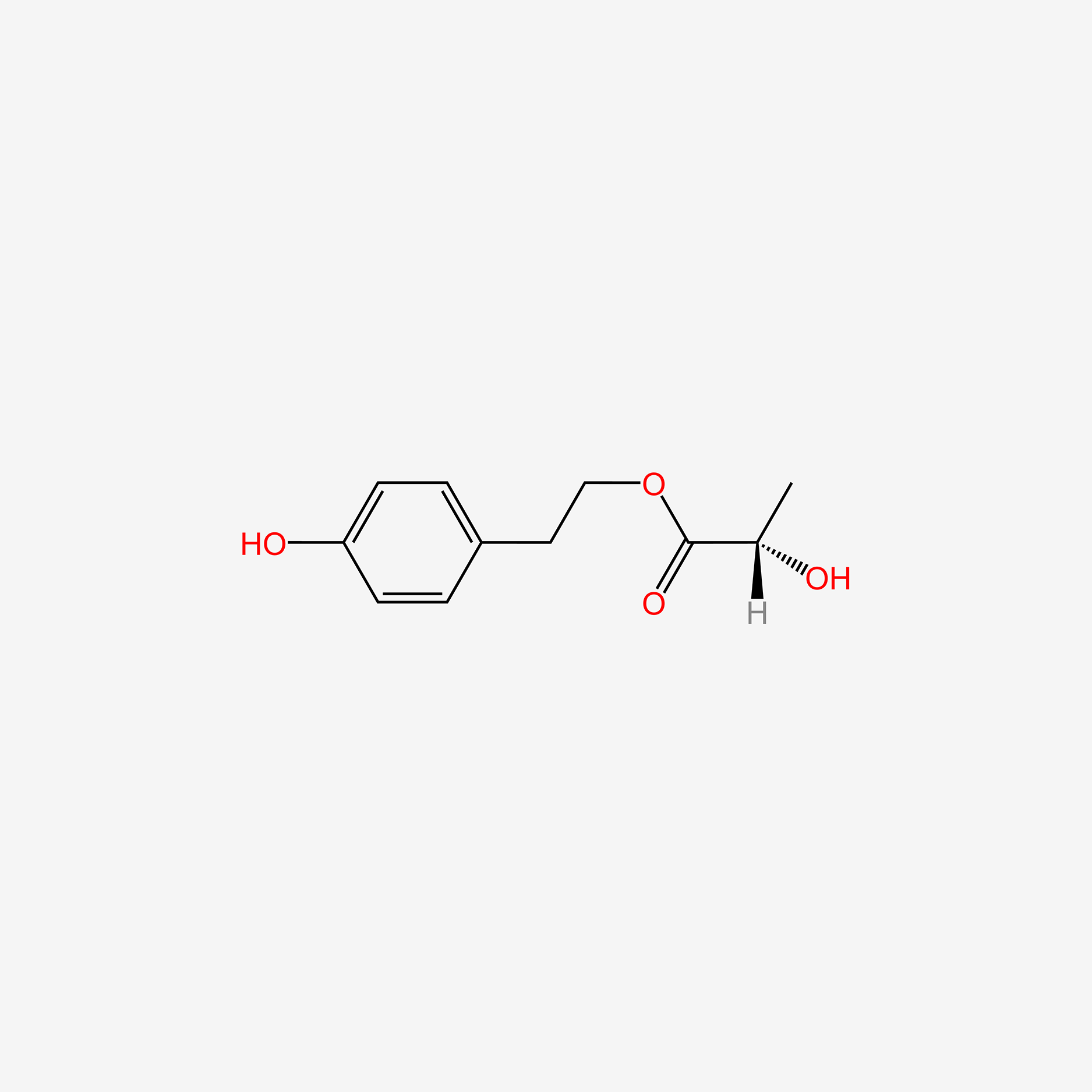 |
0.540 | D02AQY | 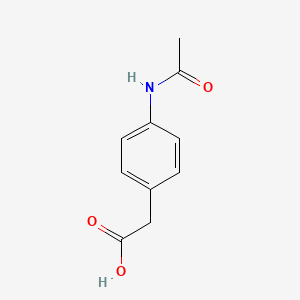 |
0.423 | ||
| ENC005811 | 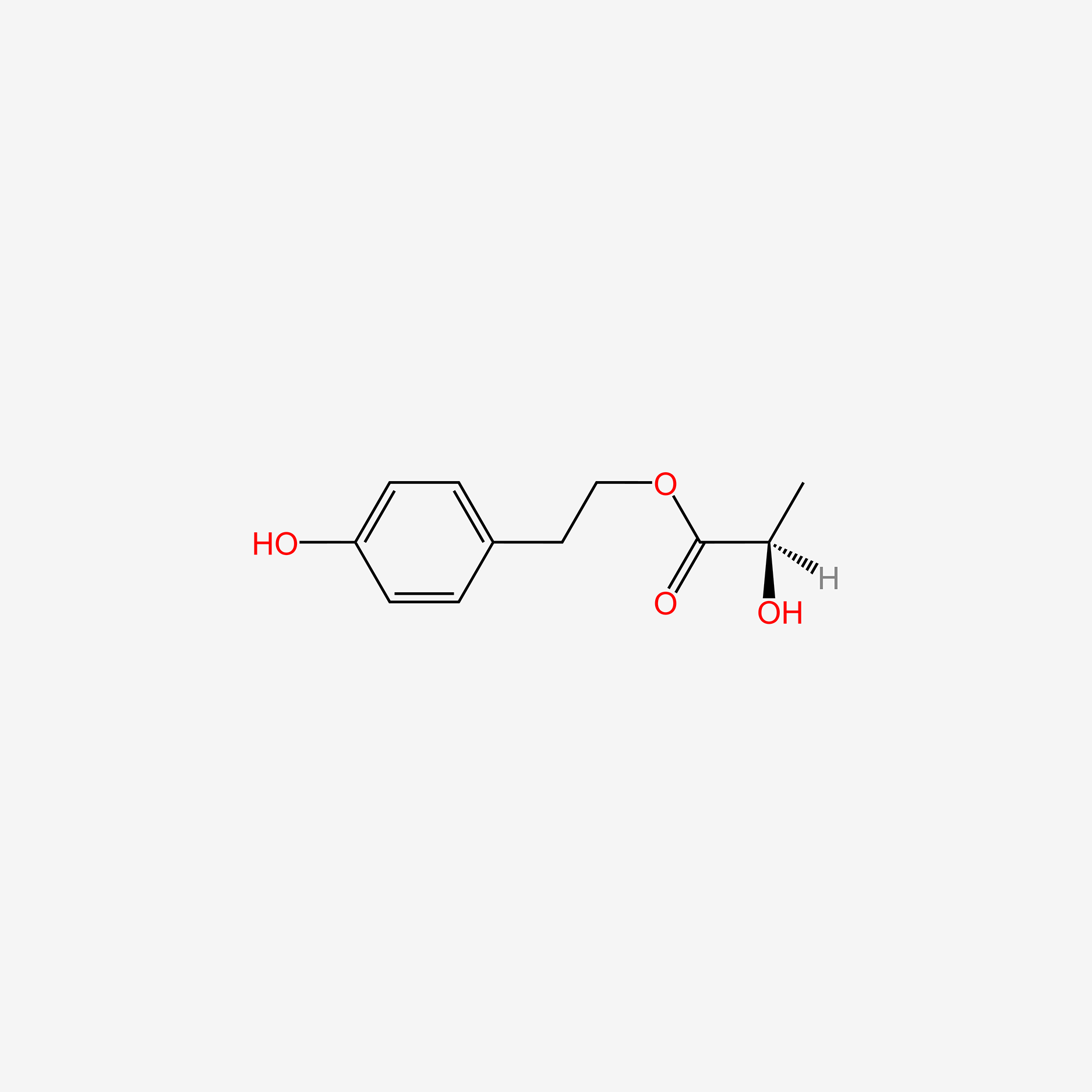 |
0.540 | D00LFB | 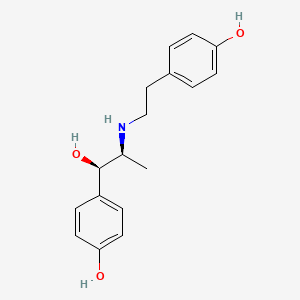 |
0.397 | ||
| ENC000774 | 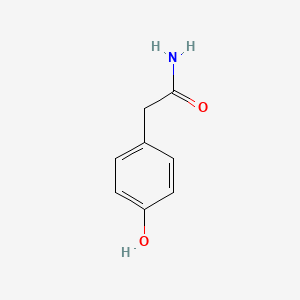 |
0.535 | D03UOT |  |
0.381 | ||
| ENC000006 | 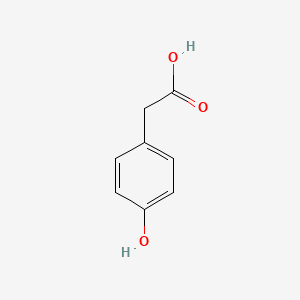 |
0.535 | D0J7RK | 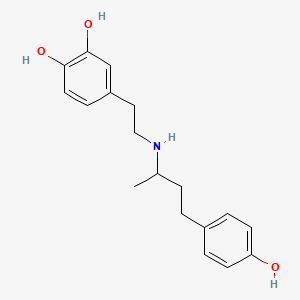 |
0.380 | ||
| ENC000072 | 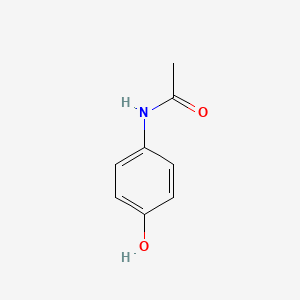 |
0.535 | D02WAB | 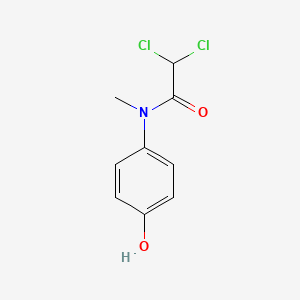 |
0.377 | ||
| ENC004860 | 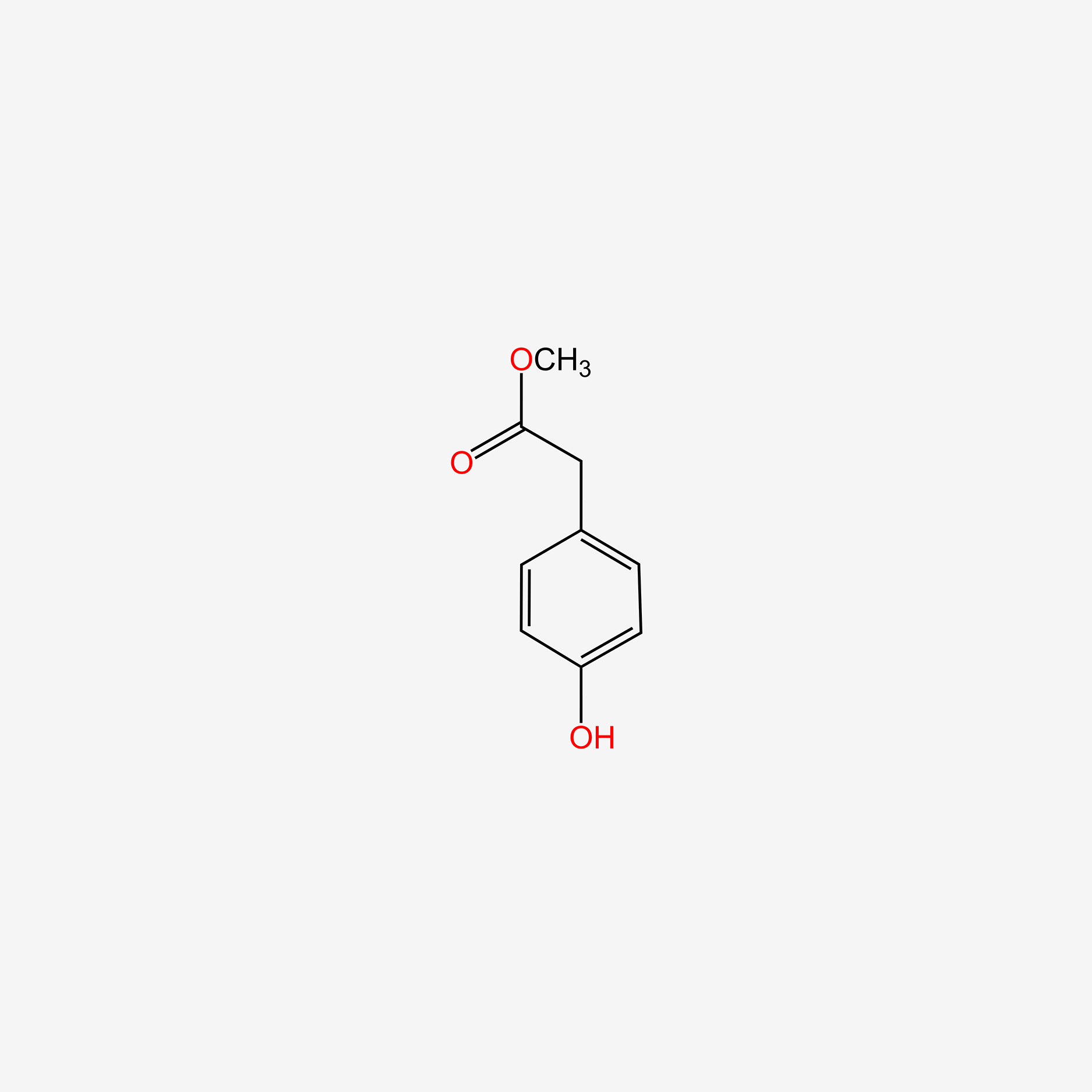 |
0.533 | D0AN7B | 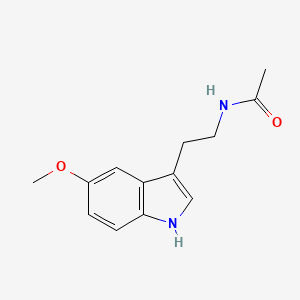 |
0.377 | ||