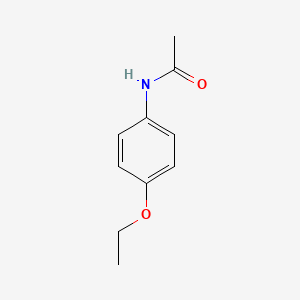
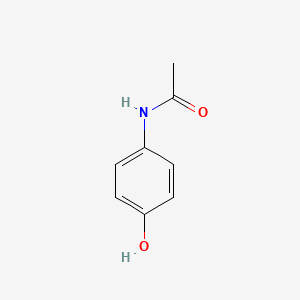
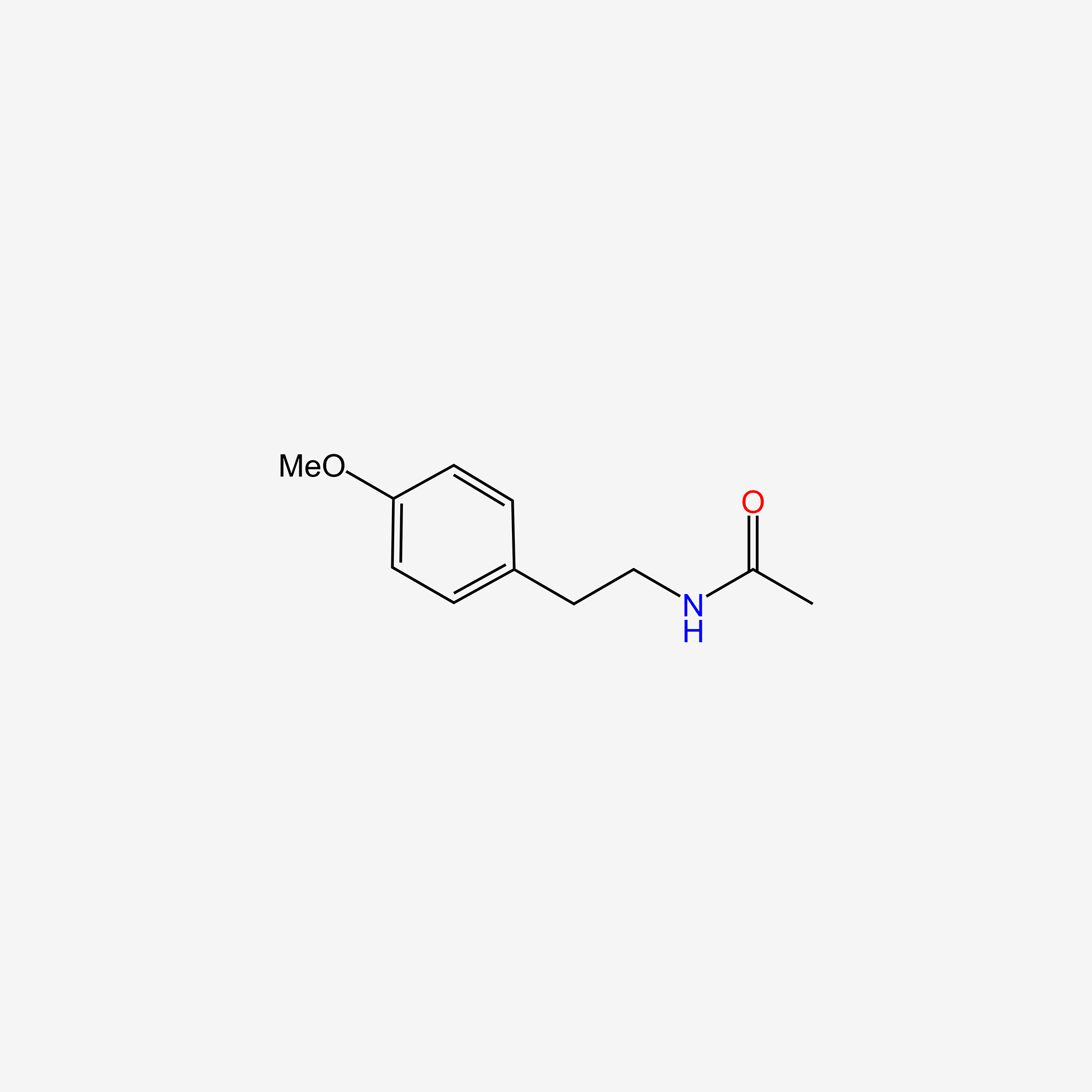
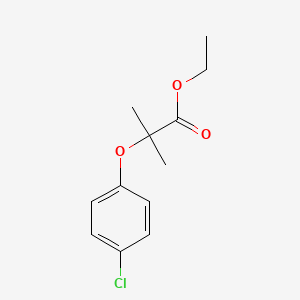
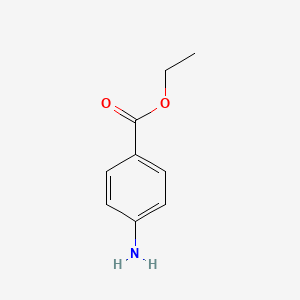
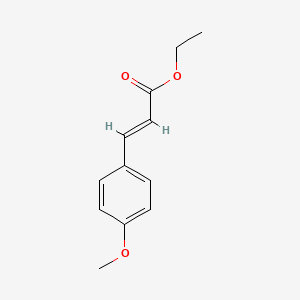
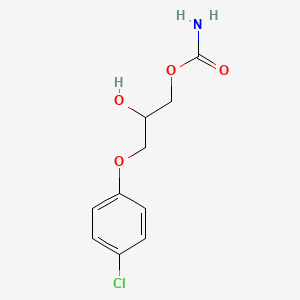
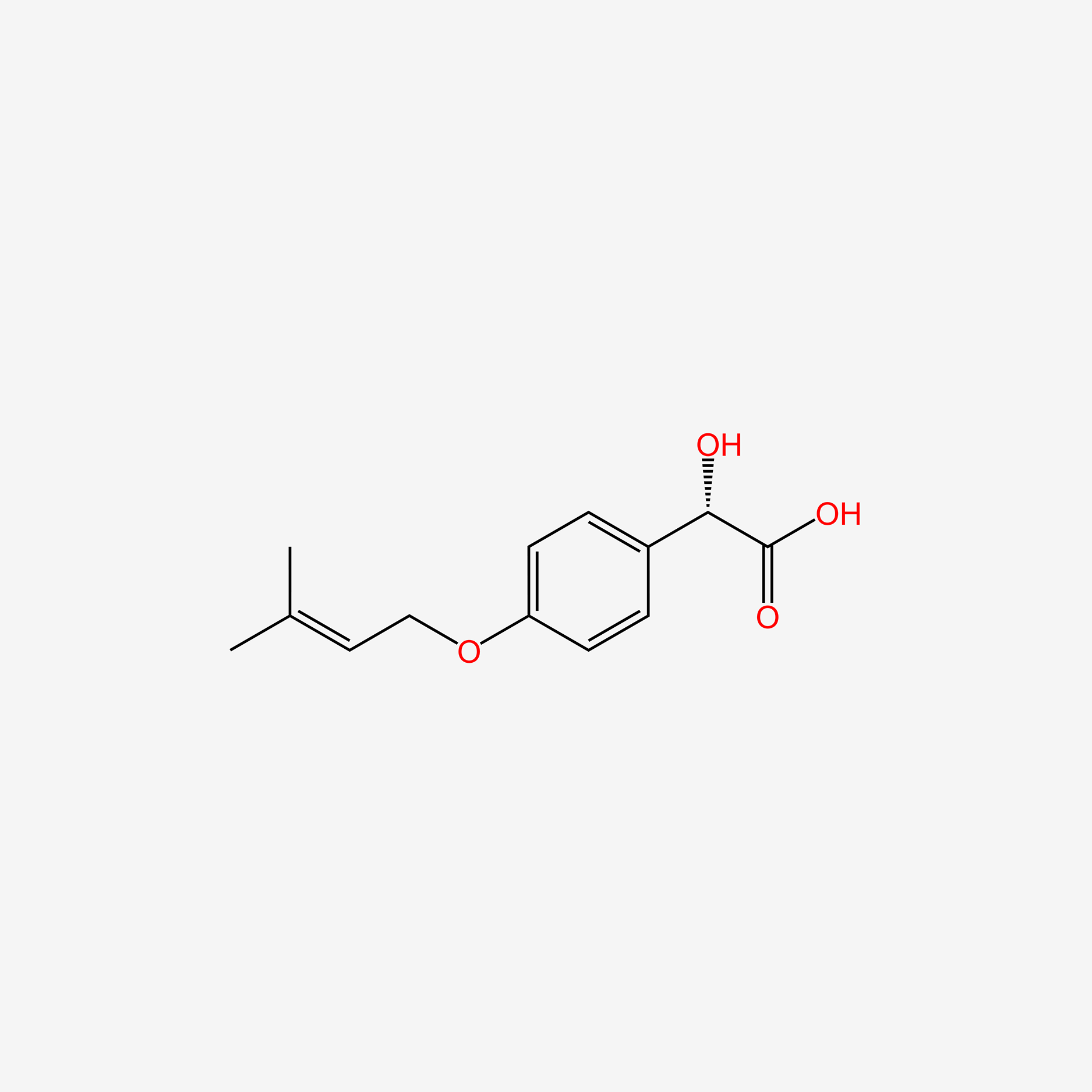
| InChI |
InChI=1S/C10H13NO2/c1-3-13-10-6-4-9(5-7-10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12)
|
| Synonyms |
phenacetin; N-(4-Ethoxyphenyl)acetamide; 62-44-2; Acetophenetidin; Acetphenetidin; Acetophenetidine; Acetophenetin; Phenacetine; p-Acetophenetidide; Phenazetin; Achrocidin; Phenacetinum; Fenidina; Kalmin; 4'-Ethoxyacetanilide; Contradouleur; Codempiral; Commotional; Contradol; 4-Ethoxyacetanilide; Acetamide, N-(4-ethoxyphenyl)-; p-Acetophenetidine; Fenacetina; Pertonal; Phenacet; Phenacitin; Phenedina; Phenidin; Pyraphen; Fenina; p-Ethoxyacetanilide; Phenin; p-Acetophenetide; p-Acetphenetidin; Phenazetina; Tetracydin; Clistanol; Coriforte; Daprisal; Dasikon; Dolostop; Edrisal; Empiral; Emprazil; Epragen; Fortacyl; Gelonida; Gewodin; Helvagit; Hocophen; Melabon; Melaforte; Pamprin; Paramette; Paratodol; Phenodyne; Pyrroxate; Quadronal; Salgydal; Sanalgine; Saridon; Seranex; Sinedal; Sinubid; Sinutab; Stellacyl; Synalogos; Treupel; Veganine; Anapac; Fenia; Malex; Tacol; Viden; Xaril; Acetylphenetidin; para-Phenacetin; Bromo seltzer; Kafa; Phenaphen plus; Robaxisal-ph; Aceto-4-phenetidine; Citra-fort; Super Anahist; Dasin ch; Emprazil-C; Acet-p-phenalide; Coryban-D; Paracetophenetidin; Hjorton'S powder; Acet-p-phenetidin; Thephorin A-C; N-Acetyl-p-phenetidine; Buff-A-Comp; Para-acetphenetidin; 1-Acetamido-4-ethoxybenzene; Fiorinal; Sinutabs; Aceto-para-phenalide; para-Acetophenetidide; Darvon compound; Acetanilide, 4'-ethoxy-; Synalgos-dc; p-Phenetidine, N-acetyl-; Aceto-para-phenetidide; Fenacetin [Czech]; Rcra waste number U187; para-Acetophenetidine; Fenacetin; para-Ethoxyacetanilide; Fenacetina [INN-Spanish]; Acetamide, N-(4-ethoxyphenol)-; N-para-Ethoxyphenylacetamide; CCRIS 496; HSDB 3152; N-Acetyl-4-ethoxyaniline; p-Ethoxyanilid kyseliny octove; p-Ethoxyanilid kyseliny octove [Czech]; BRN 1869238; Acetic acid amide, N-(4-ethoxyphenyl)-; AI3-00783; NSC-7651; 4-(Acetylamino)phenetole; ER0CTH01H9; Dolviran; 4-Ethoxy-1-acetylaminobenzene; CHEBI:8050; 69323-74-6; N-[4-(ethyloxy)phenyl]acetamide; Phenacetin Melting Point Standard; 40674-52-0; CAS-62-44-2; NCGC00016281-06; DSSTox_CID_1116; DSSTox_RID_75948; DSSTox_GSID_21116; Acetamide, N-(4-ethoxyphenyl)-, labeled with tritium; N-Acetyl-para-phenetidine; Phenacetine [INN-French]; Phenacetinum [INN-Latin]; SMR000752916; 1-Acetyl-p-phenetidin; SR-01000787183; NSC 7651; EINECS 200-533-0; RCRA waste no. U187; UNII-ER0CTH01H9; Terracydin; Phenacetin [USP:INN:JAN]; N-(4-ethoxyphenyl)-acetamide; Zactirin compound; p-Acetophenetitide; Prestwick_862; Phenacetin, 97%; 4-Ethoxy-acetanilid; N-acetylphenetylamine; MFCD00009094; 4-Ethoxy-acetanilide; ASA COMPOUND; Butigetic (Salt/Mix); Spectrum_000782; Acetanilide, p-ethoxy-; PHENACETIN [MI]; Phenacetin (JAN/INN); Phenacetin-ethoxy-[d5]; N-Acetyl-p-ethoxyaniline; PHENACETIN [INN]; PHENACETIN [JAN]; Prestwick0_000533; Prestwick1_000533; Prestwick2_000533; Prestwick3_000533; Spectrum2_001940; Spectrum3_001404; Spectrum4_000515; Spectrum5_001902; PHENACETIN [HSDB]; PHENACETIN [IARC]; PHENACETIN [INCI]; p-Acetophenetidide (8CI); PHENACETIN [VANDF]; PHENACETINUM [HPUS]; PHENACETIN [MART.]; WLN: 2OR DMV1; PHENACETIN [USP-RS]; PHENACETIN [WHO-DD]; SCHEMBL23280; 4'-Ethoxyacetanilide, 97%; BSPBio_000545; BSPBio_003048; KBioGR_001089; KBioSS_001262; N-(4-ethoxyphenyl)ethanamide; ZINC602; MLS001304971; MLS002153862; MLS002303055; CHEMBL16073; DivK1c_000580; P-A-C Compound (Salt/Mix); SPECTRUM1500642; SPBio_001979; SPBio_002466; BPBio1_000601; GTPL7402; DTXSID1021116; SCHEMBL20476396; HMS501M22; KBio1_000580; KBio2_001262; KBio2_003830; KBio2_006398; KBio3_002268; NSC7651; NINDS_000580; HMS1569L07; HMS1921M21; HMS2092E14; HMS2096L07; HMS2234P11; HMS3373I02; HMS3651N07; HMS3713L07; HMS3884H10; Pharmakon1600-01500642; BCP09084; HY-B0476; Phenacetin, >=98.0% (HPLC); Tox21_110347; Tox21_201926; Tox21_302895; BDBM50420191; CCG-39439; NSC757401; STK011463; AKOS000370201; Phenacetin 1.0 mg/ml in Acetonitrile; Tox21_110347_1; DB03783; NSC-757401; IDI1_000580; Acetamide, N-(4-ethoxyphenyl)- (9CI); NCGC00016281-01; NCGC00016281-02; NCGC00016281-03; NCGC00016281-04; NCGC00016281-05; NCGC00016281-07; NCGC00016281-08; NCGC00016281-11; NCGC00091376-01; NCGC00091376-02; NCGC00091376-03; NCGC00091376-04; NCGC00091376-05; NCGC00256345-01; NCGC00259475-01; AC-28909; SBI-0051571.P002; DB-054164; Phenacetin, Vetec(TM) reagent grade, 98%; AB00052135; FT-0631277; FT-0673664; P1669; S2577; SW196989-3; BIM-0051571.0001; C07591; D00569; D84419; EN300-178258; AB00052135_10; AB00052135_11; A833774; AE-848/04969036; Q419175; Phenacetin (136 degrees C) Melting Point Standard; SR-01000787183-2; SR-01000787183-3; BRD-K38323065-001-05-8; BRD-K38323065-001-09-0; Z27807932; Acetophenetidine Acetophenidin Acetylphenetidine Phenacetin; Phenacetin, United States Pharmacopeia (USP) Reference Standard; Phenacetin Melting Point Standard, United States Pharmacopeia (USP) Reference Standard; N4E; Phenacetin melting point standard, Pharmaceutical Secondary Standard; Certified Reference Material
|