NPs Basic Information
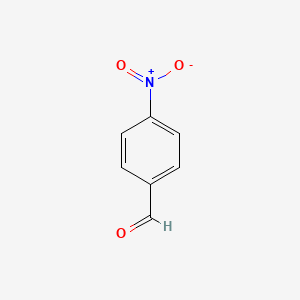
|
Name |
4-Nitrobenzaldehyde
|
| Molecular Formula | C7H5NO3 | |
| IUPAC Name* |
4-nitrobenzaldehyde
|
|
| SMILES |
C1=CC(=CC=C1C=O)[N+](=O)[O-]
|
|
| InChI |
InChI=1S/C7H5NO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5H
|
|
| InChIKey |
BXRFQSNOROATLV-UHFFFAOYSA-N
|
|
| Synonyms |
4-nitrobenzaldehyde; 555-16-8; p-Nitrobenzaldehyde; Benzaldehyde, 4-nitro-; p-Formylnitrobenzene; Benzaldehyde, p-nitro-; 4-Nitro-benzaldehyde; p-nitro benzaldehyde; 4-FORMYLNITROBENZENE; 4-nitro benzaldehyde; para-nitrobenzaldehyde; MFCD00007346; NX859P8MB0; CHEBI:66926; NSC-6103; 4-Nitrobenzaldehydde; CCRIS 1675; NSC 6103; EINECS 209-084-5; AI3-52475; 4-nitrobenzaldhyde; paranitrobenzaldehyde; XXH; p-nitro-benzaldehyde; 4-nitrobenz aldehyde; p-nitrobenzenealdehyde; para-nitro benzaldehyde; Para Nitro Benzaldehyde; (4 nitrophenyl)methanone; 4NBZ; WLN: WNR DVH; 4-Nitrobenzaldehyde,(S); DSSTox_CID_2061; 4-Nitrobenzaldehyde, 98%; SCHEMBL1157; DSSTox_RID_76477; UNII-NX859P8MB0; DSSTox_GSID_22061; NITROBENZALDEHYDE, 4-; CCRIS-1675; CHEMBL164236; DTXSID5022061; NSC6103; ZINC164513; 4-NITROBENZENECARBOXALDEHYDE; ACT07089; BCP27111; STR00898; Tox21_202930; BBL011957; STK199266; 4-Nitrobenzaldehyde, p.a., 98.0%; AKOS000118887; CS-W007577; NCGC00260476-01; AC-27489; BP-11799; CAS-555-16-8; SY001417; DB-024132; FT-0619200; FT-0672749; N0559; EN300-18420; A15647; BENZALDEHYDE,4-NITRO MFC7 H5 N1 O3; D70831; 4-Nitrobenzaldehyde, purum, >=97.0% (HPLC); AB-131/40217801; 4-Nitrobenzaldehyde, Vetec(TM) reagent grade, 98%; J-515873; Q2816679; Z57772464; F2190-0630; 4-Nitrobenzaldehyde, for spectrophotometric det. of amino sugars, >=99.0%
|
|
| CAS | 555-16-8 | |
| PubChem CID | 541 | |
| ChEMBL ID | CHEMBL164236 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 151.12 | ALogp: | 1.6 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 62.9 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.368 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.348 | MDCK Permeability: | 0.00024793 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.846 | Plasma Protein Binding (PPB): | 75.33% |
| Volume Distribution (VD): | 0.829 | Fu: | 17.29% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.914 | CYP1A2-substrate: | 0.078 |
| CYP2C19-inhibitor: | 0.243 | CYP2C19-substrate: | 0.112 |
| CYP2C9-inhibitor: | 0.031 | CYP2C9-substrate: | 0.773 |
| CYP2D6-inhibitor: | 0.017 | CYP2D6-substrate: | 0.403 |
| CYP3A4-inhibitor: | 0.028 | CYP3A4-substrate: | 0.19 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.508 | Half-life (T1/2): | 0.477 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.286 | Human Hepatotoxicity (H-HT): | 0.182 |
| Drug-inuced Liver Injury (DILI): | 0.22 | AMES Toxicity: | 0.982 |
| Rat Oral Acute Toxicity: | 0.061 | Maximum Recommended Daily Dose: | 0.071 |
| Skin Sensitization: | 0.921 | Carcinogencity: | 0.714 |
| Eye Corrosion: | 0.985 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.976 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000005 |  |
0.528 | D0X6IU |  |
0.361 | ||
| ENC000026 |  |
0.463 | D0I8DD | 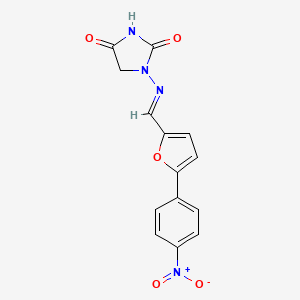 |
0.319 | ||
| ENC000672 | 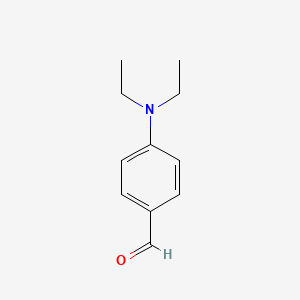 |
0.404 | D0E9CD |  |
0.304 | ||
| ENC000122 | 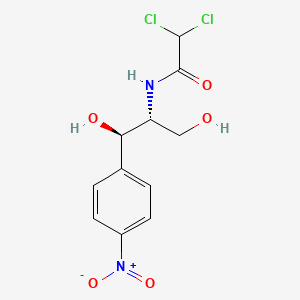 |
0.361 | D0J9ZR | 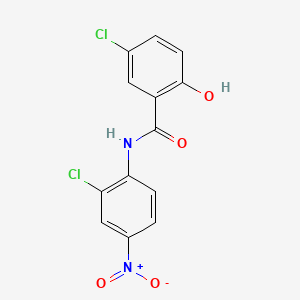 |
0.299 | ||
| ENC000793 | 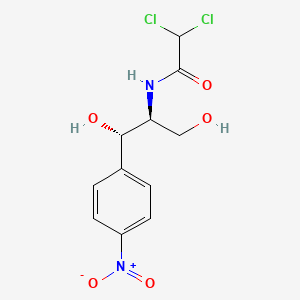 |
0.361 | D0T5WK | 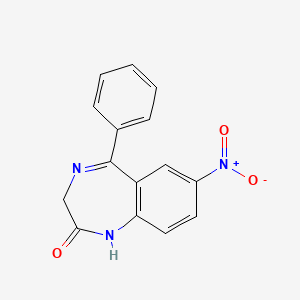 |
0.286 | ||
| ENC000012 | 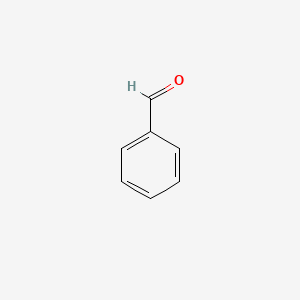 |
0.359 | D0CP4E | 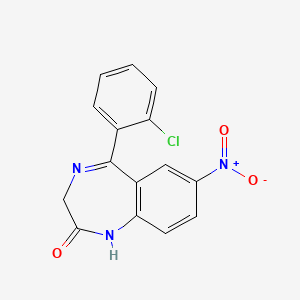 |
0.278 | ||
| ENC000414 | 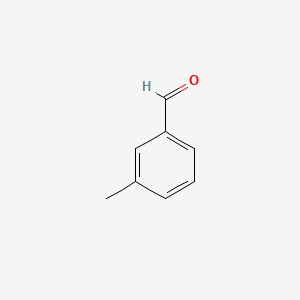 |
0.341 | D05HFY | 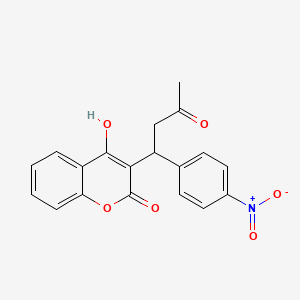 |
0.275 | ||
| ENC001381 | 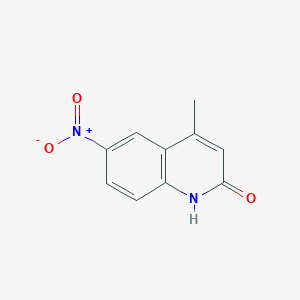 |
0.340 | D0W2NM | 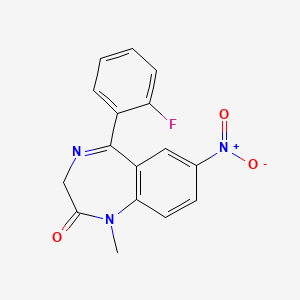 |
0.270 | ||
| ENC001854 | 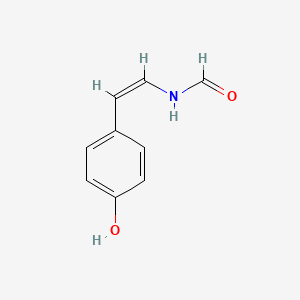 |
0.333 | D0IE1E | 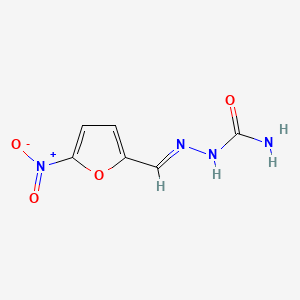 |
0.259 | ||
| ENC001676 | 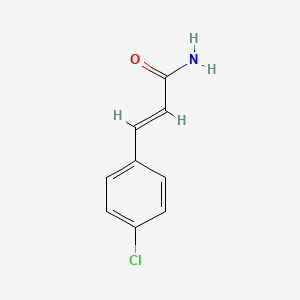 |
0.313 | D0Y7PG | 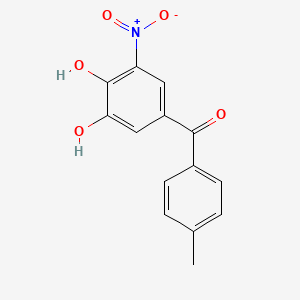 |
0.254 | ||