NPs Basic Information
|
Name |
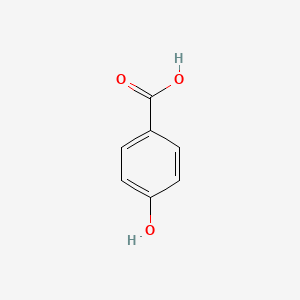
4-Hydroxybenzoic acid
|
| Molecular Formula | C7H6O3 | |
| IUPAC Name* |
4-hydroxybenzoic acid
|
|
| SMILES |
C1=CC(=CC=C1C(=O)O)O
|
|
| InChI |
InChI=1S/C7H6O3/c8-6-3-1-5(2-4-6)7(9)10/h1-4,8H,(H,9,10)
|
|
| InChIKey |
FJKROLUGYXJWQN-UHFFFAOYSA-N
|
|
| Synonyms |
4-HYDROXYBENZOIC ACID; 99-96-7; p-Hydroxybenzoic acid; 4-Carboxyphenol; p-Salicylic acid; Benzoic acid, 4-hydroxy-; Benzoic acid, p-hydroxy-; para-Hydroxybenzoic acid; p-carboxyphenol; 4-Hydroxybenzoesaeure; Paraben-acid; 4-hydroxy benzoic acid; p-Oxybenzoesaure [German]; 4-Hydroxybenzoicacid; PHBA; 4-hydroxy-benzoic acid; Hydroxybenzoic acid; Acido p-idrossibenzoico [Italian]; Kyselina 4-hydroxybenzoova; Kyselina 4-hydroxybenzoova [Czech]; NSC 4961; parahydroxybenzoic acid; HYDROXYBENZOIC ACID, PARA; p-hydroxy benzoic acid; p-hydroxy-Benzoic acid; Hydroxybenzenecarboxylic acid; 4-HBA; HSDB 7233; CHEMBL441343; JG8Z55Y12H; EINECS 202-804-9; CHEBI:30763; NSC-4961; MFCD00002547; AI3-01003; 3pcc; 3pch; 4-hydroxy-benzoate; DSSTox_CID_6647; DSSTox_RID_78173; DSSTox_GSID_26647; 30729-36-3; Benzoic acid, p-hydroxy; Benzoic acid, 4-hydroxy; WLN: QVR DQ; p-Oxybenzoesaure; CAS-99-96-7; NSC4961; Acido p-idrossibenzoico; DB04242; NCGC00166040-01; PHB; UNII-JG8Z55Y12H; C00156; p-Salicylate; AE-848/32195059; CCRIS 8812; p-hydroxy-Benzoate; para-salicylic acid; 4-hydoxybenzoic acid; 4-hyroxybenzoic acid; phenol derivative, 8; 4-hydroxylbenzoic acid; 4-Hydroxy-benzoesaeure; 4-hydroxybenzoi c acid; 4-hydroxyl benzoic acid; 4-Hydroxybenzoate, III; para-hydroxy benzoic acid; Para Hydroxy Benzoic Acid; bmse000092; bmse000583; EC 202-804-9; SCHEMBL4110; BIDD:ER0706; 4-Hydroxybenzenecarboxylic acid; p-Hydroxybenzoic Acid, Reagent; DTXSID3026647; FEMA NO. 3986; BDBM26194; LR-68; ZINC332752; P-HYDROXYBENZOIC ACID [MI]; CS-D1180; HY-Y0264; STR01287; Tox21_202342; Tox21_303301; AC-008; BBL011981; s3754; STL138745; 4-HYDROXYBENZOIC ACID [FHFI]; 4-HYDROXYBENZOIC ACID [HSDB]; 4-HYDROXYBENZOIC ACID [INCI]; 4-Hydroxybenzoic acid, >=99%, FG; AKOS000119033; AM87513; CCG-266143; NCGC00166040-02; NCGC00257058-01; NCGC00259891-01; FT-0618695; FT-0669322; H0207; 4-Hydroxybenzoic acid, ReagentPlus(R), 99%; EN300-21461; D86505; SALICYLIC ACID IMPURITY A [EP IMPURITY]; 4-Hydroxybenzoic acid, ReagentPlus(R), >=99%; 4-Hydroxybenzoic acid, puriss., >=99.0% (T); A858402; Q229970; 46DD083D-BFD3-4CE1-B2D9-6C6D5FEFD3D9; J-660066; W-100004; 4-Hydroxybenzoic acid, Vetec(TM) reagent grade, 99%; ACETYLSALICYLIC ACID IMPURITY A [EP IMPURITY]; PROPYL HYDROXYBENZOATE IMPURITY A [EP IMPURITY]; F2191-0237; Z104498098; METHYL PARAHYDROXYBENZOATE IMPURITY A [EP IMPURITY]; 4-Hydroxybenzoic acid, certified reference material, TraceCERT(R); 4-Hydroxybenzoic acid, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 99-96-7 | |
| PubChem CID | 135 | |
| ChEMBL ID | CHEMBL441343 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 138.12 | ALogp: | 1.6 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.618 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.27 | MDCK Permeability: | 0.00000849 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.01 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.308 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.314 | Plasma Protein Binding (PPB): | 38.35% |
| Volume Distribution (VD): | 0.291 | Fu: | 49.50% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.034 | CYP1A2-substrate: | 0.072 |
| CYP2C19-inhibitor: | 0.04 | CYP2C19-substrate: | 0.047 |
| CYP2C9-inhibitor: | 0.033 | CYP2C9-substrate: | 0.178 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.118 |
| CYP3A4-inhibitor: | 0.038 | CYP3A4-substrate: | 0.062 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.575 | Half-life (T1/2): | 0.924 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.048 | Human Hepatotoxicity (H-HT): | 0.449 |
| Drug-inuced Liver Injury (DILI): | 0.794 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.531 | Maximum Recommended Daily Dose: | 0.004 |
| Skin Sensitization: | 0.247 | Carcinogencity: | 0.05 |
| Eye Corrosion: | 0.173 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.37 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000665 | 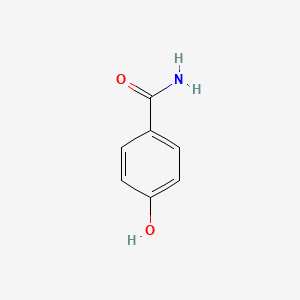 |
0.688 | D03UOT |  |
0.531 | ||
| ENC000200 | 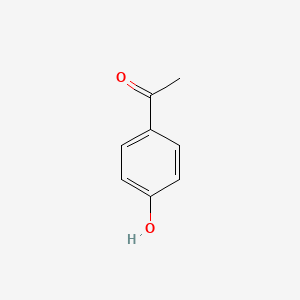 |
0.688 | D01CRB | 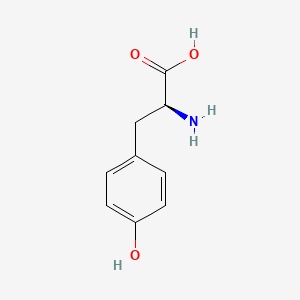 |
0.512 | ||
| ENC002802 | 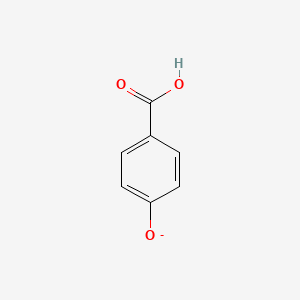 |
0.636 | D0U5QK | 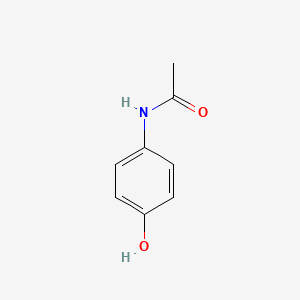 |
0.500 | ||
| ENC000195 | 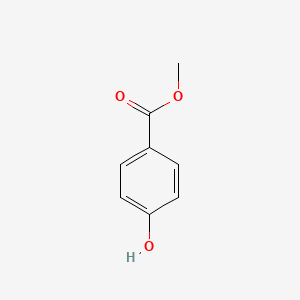 |
0.629 | D0B3QM | 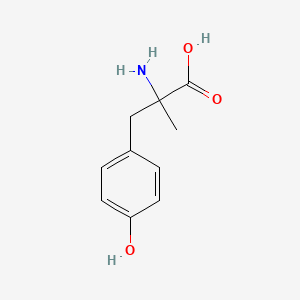 |
0.488 | ||
| ENC005097 | 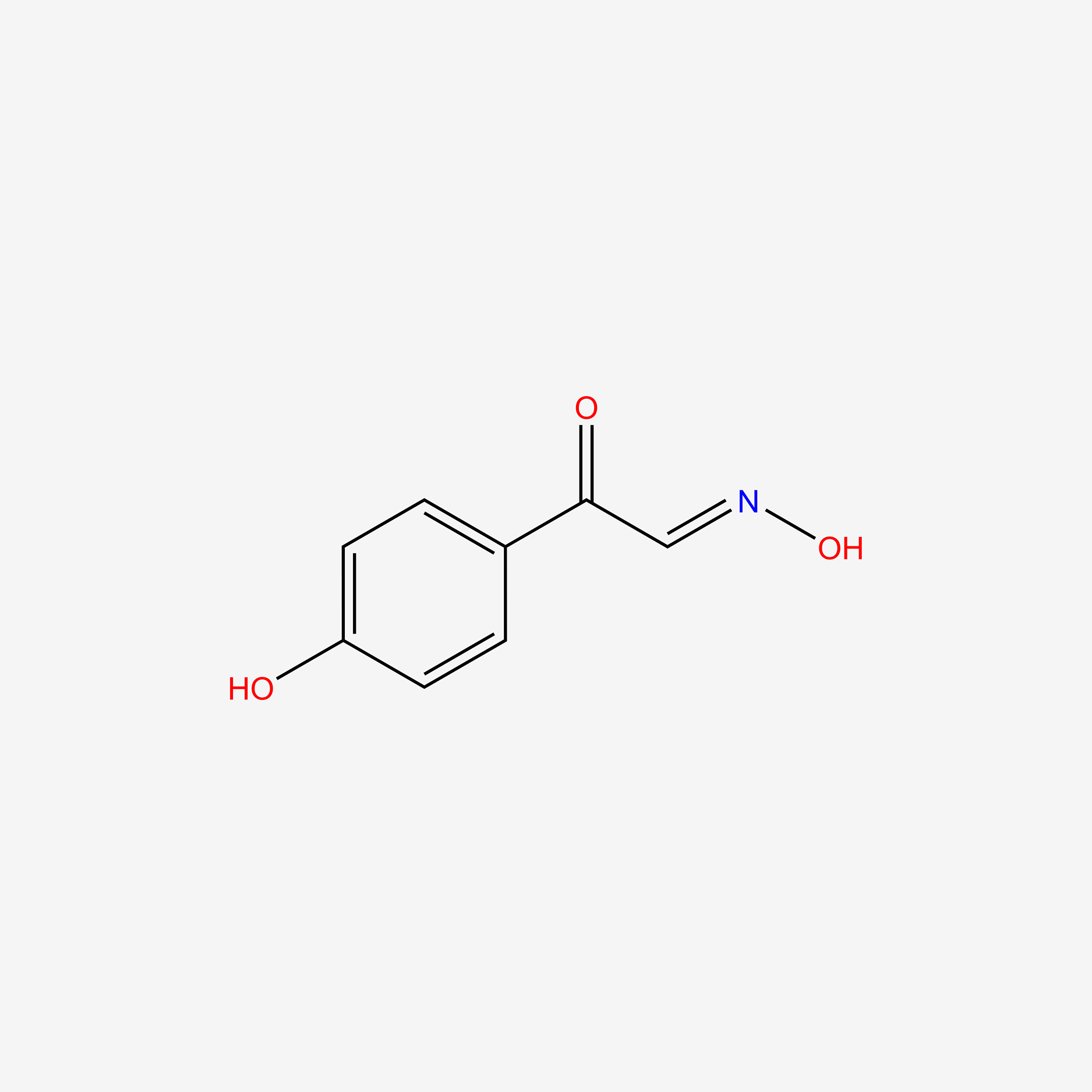 |
0.622 | D02WAB | 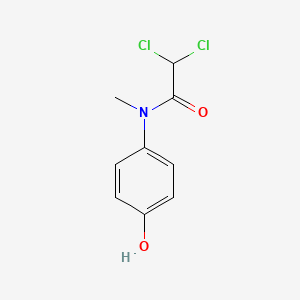 |
0.422 | ||
| ENC000202 | 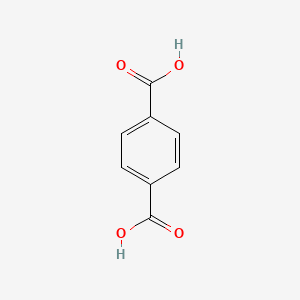 |
0.595 | D07HBX |  |
0.421 | ||
| ENC000006 | 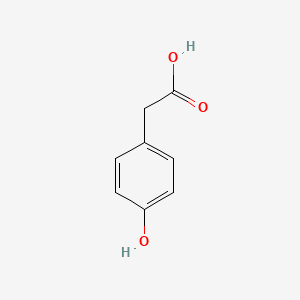 |
0.583 | D0W1RY | 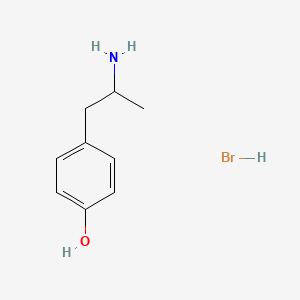 |
0.381 | ||
| ENC001420 | 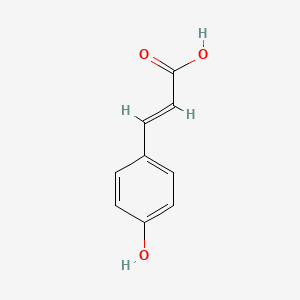 |
0.538 | D0L7FM | 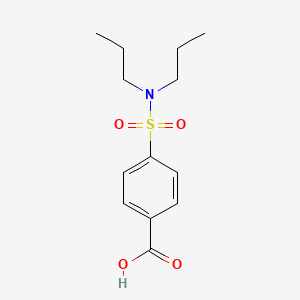 |
0.368 | ||
| ENC000129 | 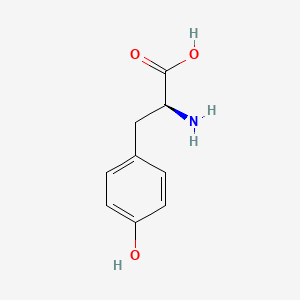 |
0.512 | D06NVJ | 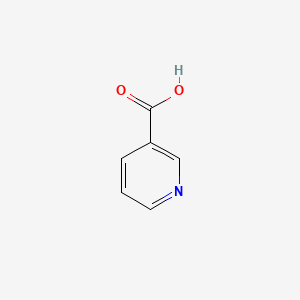 |
0.368 | ||
| ENC000774 | 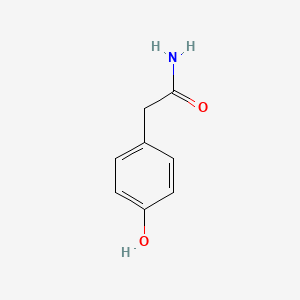 |
0.500 | D0C4YC | 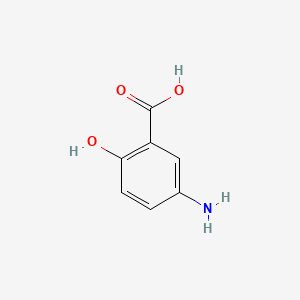 |
0.366 | ||