NPs Basic Information
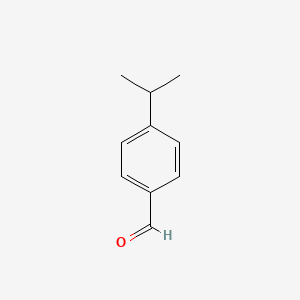
|
Name |
4-Isopropylbenzaldehyde
|
| Molecular Formula | C10H12O | |
| IUPAC Name* |
4-propan-2-ylbenzaldehyde
|
|
| SMILES |
CC(C)C1=CC=C(C=C1)C=O
|
|
| InChI |
InChI=1S/C10H12O/c1-8(2)10-5-3-9(7-11)4-6-10/h3-8H,1-2H3
|
|
| InChIKey |
WTWBUQJHJGUZCY-UHFFFAOYSA-N
|
|
| Synonyms |
4-isopropylbenzaldehyde; cuminaldehyde; 122-03-2; cuminic aldehyde; cumaldehyde; cuminal; p-cumic aldehyde; Cumic aldehyde; Benzaldehyde, 4-(1-methylethyl)-; Cuminyl aldehyde; p-Isopropylbenzenecarboxaldehyde; P-ISOPROPYLBENZALDEHYDE; 4-(1-Methylethyl)benzaldehyde; Benzaldehyde, p-isopropyl-; p-Cuminic aldehyde; P-isopropyl benzaldehyde; 4-(Propan-2-Yl)Benzaldehyde; 4-propan-2-ylbenzaldehyde; 4-Isopropylbenzenecarboxylate; FEMA No. 2341; 4-Isopropyl-benzaldehyde; NSC 4886; MFCD00006953; CHEMBL161577; CHEBI:28671; O0893NC35F; NSC-4886; 4-(Methylethyl)benzaldehyde; EINECS 204-516-9; BRN 0636547; Cuminadlehyde; Cuminaldehyd; Cumal; Cumin aldehyde; UNII-O0893NC35F; AI3-01853; 4-iPr-Benzaldehyde; 4isopropylbenzaldehyde; Cuminaldehyde, 98%; 4-i-propylbenzaldehyde; p-iso-Propylbenzaldehyde; p-isopropyl-Benzaldehyde; 4(isopropyl)benzaldehyde; 4-isopropyl benzaldehyde; 4-(isopropyl)benzaldehyde; DSSTox_CID_1974; p-Isopropylbenzaldehyde, f; 4(2-propyl)-benzaldehyde; CUMINALDEHYDE [MI]; bmse000508; EC 204-516-9; CUMINALDEHYDE [FHFI]; DSSTox_RID_76435; DSSTox_GSID_21974; SCHEMBL87226; 4-07-00-00723 (Beilstein Handbook Reference); p-(1-methylethyl)benzaldehyde; 4-Isopropylphenylcarboxaldehyde; CUMINIC ALDEHYDE [FCC]; (4-isopropyl-phenyl)-methanone; DTXSID9021974; WLN: VHR DY1 & 1; NSC4886; Cuminaldehyde, analytical standard; ZINC968248; HY-Y0790; Cuminaldehyde, >=98%, FCC, FG; Tox21_300712; BDBM50139366; s5089; STL194065; AKOS000119738; AC-2430; CCG-266191; Cuminal p-(1-methylethyl)benzaldehyde; NCGC00248148-01; NCGC00257518-01; AS-12957; CAS-122-03-2; DB-041645; CS-0015770; FT-0624115; I0168; EN300-19901; C06577; D70801; A804831; Q419952; W-108440; F2190-0632; Z104476006
|
|
| CAS | 122-03-2 | |
| PubChem CID | 326 | |
| ChEMBL ID | CHEMBL161577 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 148.2 | ALogp: | 2.7 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.587 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.363 | MDCK Permeability: | 0.00002480 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.006 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.966 | Plasma Protein Binding (PPB): | 88.64% |
| Volume Distribution (VD): | 1.177 | Fu: | 10.92% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.966 | CYP1A2-substrate: | 0.52 |
| CYP2C19-inhibitor: | 0.582 | CYP2C19-substrate: | 0.513 |
| CYP2C9-inhibitor: | 0.287 | CYP2C9-substrate: | 0.621 |
| CYP2D6-inhibitor: | 0.078 | CYP2D6-substrate: | 0.304 |
| CYP3A4-inhibitor: | 0.04 | CYP3A4-substrate: | 0.416 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.727 | Half-life (T1/2): | 0.345 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.019 |
| Drug-inuced Liver Injury (DILI): | 0.094 | AMES Toxicity: | 0.023 |
| Rat Oral Acute Toxicity: | 0.024 | Maximum Recommended Daily Dose: | 0.041 |
| Skin Sensitization: | 0.153 | Carcinogencity: | 0.248 |
| Eye Corrosion: | 0.958 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.961 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000199 | 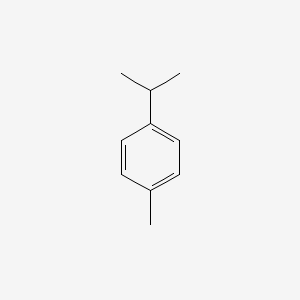 |
0.583 | D0R1QE | 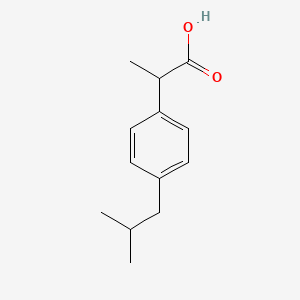 |
0.373 | ||
| ENC001121 | 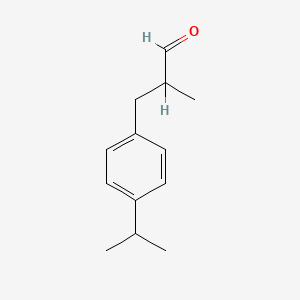 |
0.545 | D06YPU | 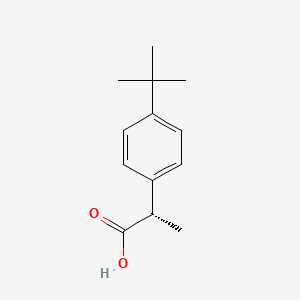 |
0.353 | ||
| ENC000005 | 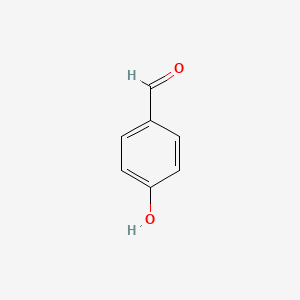 |
0.528 | D0E9CD |  |
0.333 | ||
| ENC000672 | 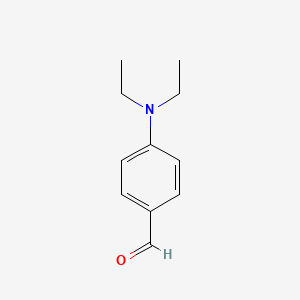 |
0.467 | D04VMT | 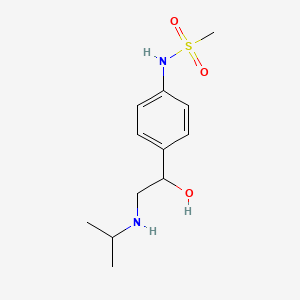 |
0.322 | ||
| ENC000034 | 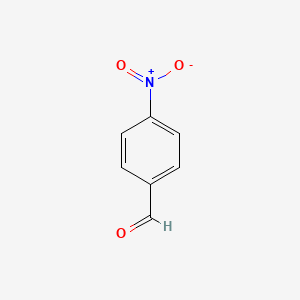 |
0.463 | D0DJ1B |  |
0.283 | ||
| ENC000191 | 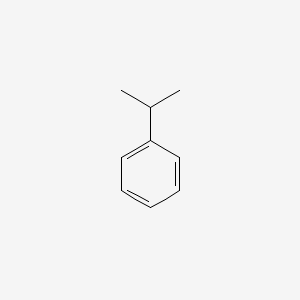 |
0.410 | D01AJY | 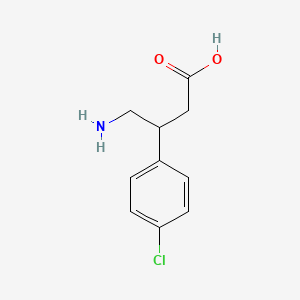 |
0.283 | ||
| ENC000368 | 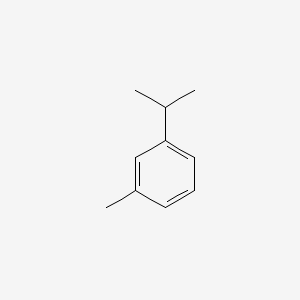 |
0.390 | D06GIP | 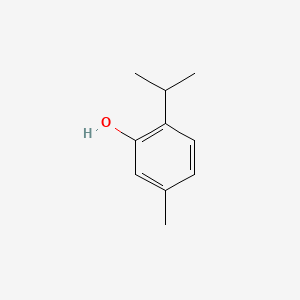 |
0.283 | ||
| ENC000414 | 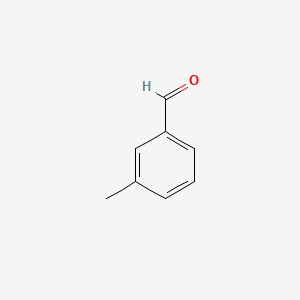 |
0.375 | D0YQ5L | 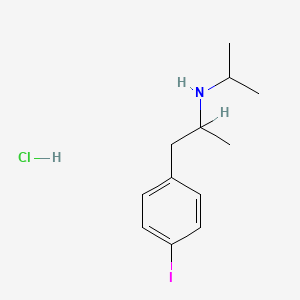 |
0.278 | ||
| ENC000347 | 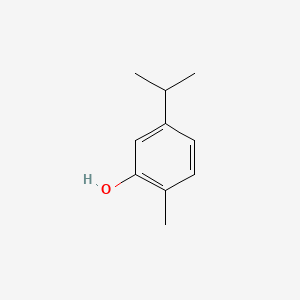 |
0.372 | D08GYO | 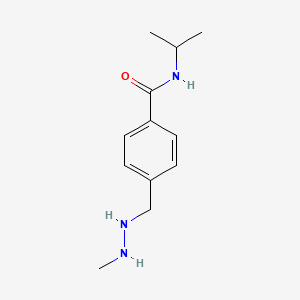 |
0.276 | ||
| ENC000098 | 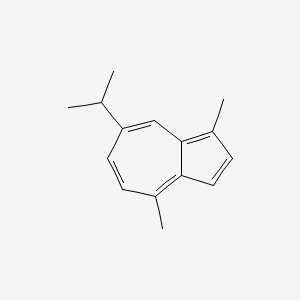 |
0.365 | D0W1RY | 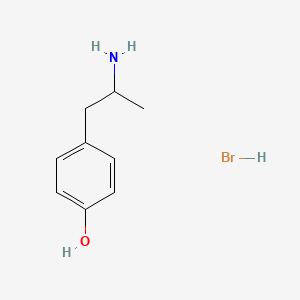 |
0.271 | ||