NPs Basic Information
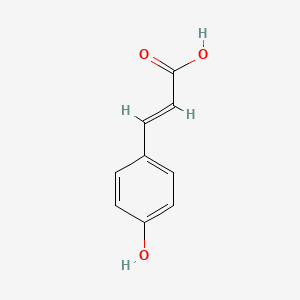
|
Name |
4-Hydroxycinnamic acid
|
| Molecular Formula | C9H8O3 | |
| IUPAC Name* |
(E)-3-(4-hydroxyphenyl)prop-2-enoic acid
|
|
| SMILES |
C1=CC(=CC=C1/C=C/C(=O)O)O
|
|
| InChI |
InChI=1S/C9H8O3/c10-8-4-1-7(2-5-8)3-6-9(11)12/h1-6,10H,(H,11,12)/b6-3+
|
|
| InChIKey |
NGSWKAQJJWESNS-ZZXKWVIFSA-N
|
|
| Synonyms |
4-Hydroxycinnamic acid; p-coumaric acid; 501-98-4; p-Hydroxycinnamic acid; 4-Coumaric acid; trans-4-Hydroxycinnamic acid; 7400-08-0; trans-p-Coumaric acid; p-Cumaric acid; 3-(4-hydroxyphenyl)acrylic acid; Para-Coumaric acid; Hydroxycinnamic acid; Naringeninic acid; p-Hydroxy-cinnamic acid; (E)-p-Coumaric acid; (E)-3-(4-Hydroxyphenyl)acrylic acid; trans-4-coumaric acid; trans-p-Coumarinic acid; 4'-hydroxycinnamic acid; 2-propenoic acid, 3-(4-hydroxyphenyl)-, (2E)-; p-Hydroxyphenylacrylic acid; Cinnamic acid, p-hydroxy-; (E)-p-Hydroxycinnamic acid; trans-p-Hydroxycinnamic acid; 3-(4-Hydroxyphenyl)-2-propenoic acid; (2E)-3-(4-hydroxyphenyl)prop-2-enoic acid; (E)-3-(4-hydroxyphenyl)prop-2-enoic acid; 4-coumarate; trans-p-Hydroxycinnamate; 2-Propenoic acid, 3-(4-hydroxyphenyl)-; 4-Hydroxycinnamate; Cinnamic acid, p-hydroxy-, (E)-; (E)-3-(4-Hydroxyphenyl)-2-propenoic acid; 3-(4-hydroxyphenyl)prop-2-enoic acid; trans-4-hydroxycinnamate; (2E)-3-(4-hydroxyphenyl)acrylic acid; beta-(4-Hydroxyphenyl)acrylic acid; Para coumaric acid; (E)-4-hydroxycinnamic acid; NSC 59260; 4-Hydroxycinamic acid; IBS9D1EU3J; 2-Propenoic acid, 3-(4-hydroxyphenyl)-, (E)-; 4-hydroxy cinnamic acid; CHEMBL66879; CHEBI:32374; beta-[4-Hydroxyphenyl]acrylic acid; NSC-59260; NSC674321; NSC-674321; 50940-26-6; trans-p-coumarate; 3-(4-hydroxyphenyl)acrylate; MFCD00004399; .beta.-[4-Hydroxyphenyl]acrylic acid; parahydroxycinnamic acid; (E)-3-[4-hydroxyphenyl]-2-propenoic acid; 4-Hydroxycinnamicacid; trans-p-Cumaric Acid; .BETA.-(4-HYDROXYPHENYL)ACRYLIC ACID; 2-Propenoic acid, 3-(4-hydroxyphenyl)-, (Z)-; EINECS 231-000-0; UNII-IBS9D1EU3J; NSC 674321; CHEBI:36090; BRN 2207381; BRN 2207383; hydroxycinnamate; Para coumarate; p-coumaric-acid; Para-Coumarate; p-Cumarate; naringeninic-acid; p-Hydroxycinnamate; 4qem; Coumaric acid, p-; 4'-Hydroxycinnamate; 4-Hydroxy cinnamate; p-Coumaric acid,trans; ORISTAR PCA; p-Coumaric acid 98%; 4f8j; p-Coumaric acid, trans; 4-Hydroxyphenylpropenoate; (2E)-3-(4-Hydroxyphenyl)-2-propenoic acid; bmse000150; bmse000591; bmse010208; trans-4-HydroxycinnamicAcid; b-[4-Hydroxyphenyl]acrylate; SCHEMBL39106; p-hydroxycinnamic acid (M4); 0-10-00-00297 (Beilstein Handbook Reference); 4-10-00-01005 (Beilstein Handbook Reference); MLS001066419; p-Hydroxycinnamic acid, trans; P-COUMARIC ACID [MI]; beta-[4-Hydroxyphenyl]acrylate; BDBM4374; GTPL5787; PARA HYDROXYCINNAMIC ACID; SODIUM2,4-PENTANEDIONATE; b-[4-Hydroxyphenyl]acrylic acid; trans-p-HydroxyzimtsA currencyure; ZINC39811; DTXSID30901076; P-COUMARIC ACID [WHO-DD]; HMS1409E10; 3-(4-Hydroxyphenyl)-2-propenoate; HYDROXYCINNAMIC ACID [INCI]; BCP22803; HY-N2391; NSC59260; STR06515; 4-HYDROXYPHENYLPROPENOIC ACID; Cinnamic acid, 4-hydroxy-, trans-; AC7957; BBL012226; CK2547; s4759; s9564; STL163567; AKOS000120685; p-Coumaric acid;p-Hydroxycinnamic acid; BCP9001042; CCG-266309; CS-W020394; DB04066; p-Coumaric acid, >=98.0% (HPLC); (E)-3-(4-hydroxyphenyl)prop-2-enoate; NCGC00246974-01; AC-10318; AC-34130; AC-34133; AS-12000; BP-13278; SMR000112201; (E)-3-(4-hydroxyphenyl)prop-2-enoicacid; trans-p-Coumaric acid, analytical standard; AM20050138; EN300-17292; A14559; A19490; C00811; Q99374; (2E)-3-(4-Hydroxyphenyl)-2-propenoic acid #; 400H080; A828008; AE-562/40414679; trans-p-Coumaric acid 1000 microg/mL in Acetone; Q-100560; W-104438; Z56911963; 0C1BFF2D-2CF7-4FC1-9F76-3268C2C7F783; F2191-0188; p-Coumaric acid, primary pharmaceutical reference standard; p-coumaric acid methyl ester geometric isomer (tentative, MSe); (E)-3-(4-hydroxyphenyl)prop-2-enoate;Trans-4-Hydroxycinnamic Acid
|
|
| CAS | 501-98-4 | |
| PubChem CID | 637542 | |
| ChEMBL ID | CHEMBL66879 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 164.16 | ALogp: | 1.5 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.657 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.961 | MDCK Permeability: | 0.00001320 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.017 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.194 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.29 | Plasma Protein Binding (PPB): | 85.36% |
| Volume Distribution (VD): | 0.293 | Fu: | 13.73% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.061 | CYP1A2-substrate: | 0.067 |
| CYP2C19-inhibitor: | 0.046 | CYP2C19-substrate: | 0.052 |
| CYP2C9-inhibitor: | 0.232 | CYP2C9-substrate: | 0.566 |
| CYP2D6-inhibitor: | 0.019 | CYP2D6-substrate: | 0.169 |
| CYP3A4-inhibitor: | 0.031 | CYP3A4-substrate: | 0.08 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.299 | Half-life (T1/2): | 0.919 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.025 | Human Hepatotoxicity (H-HT): | 0.673 |
| Drug-inuced Liver Injury (DILI): | 0.2 | AMES Toxicity: | 0.045 |
| Rat Oral Acute Toxicity: | 0.796 | Maximum Recommended Daily Dose: | 0.031 |
| Skin Sensitization: | 0.941 | Carcinogencity: | 0.151 |
| Eye Corrosion: | 0.672 | Eye Irritation: | 0.988 |
| Respiratory Toxicity: | 0.512 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001441 | 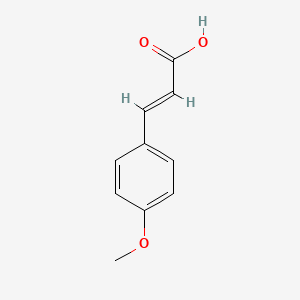 |
0.643 | D0V9EN |  |
0.545 | ||
| ENC000005 | 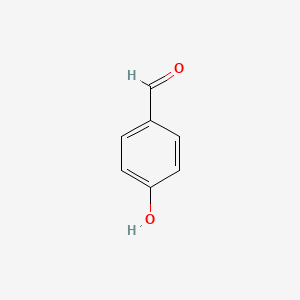 |
0.568 | D01ZJK |  |
0.524 | ||
| ENC001854 | 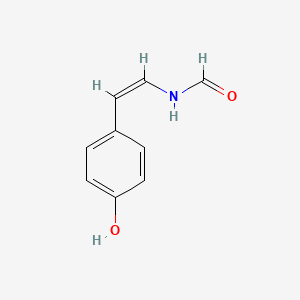 |
0.558 | D0C7AA | 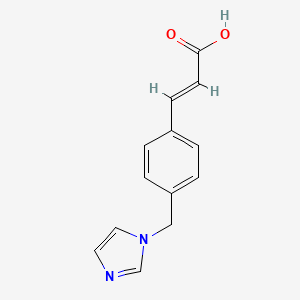 |
0.491 | ||
| ENC001440 | 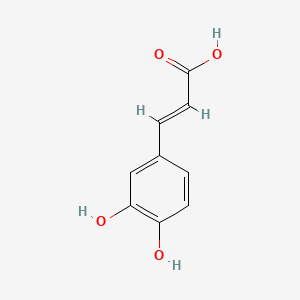 |
0.545 | D03UOT |  |
0.447 | ||
| ENC000007 | 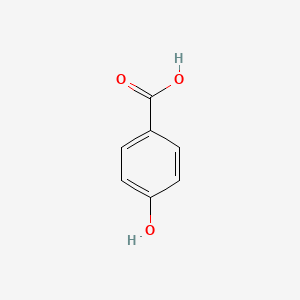 |
0.538 | D01CRB | 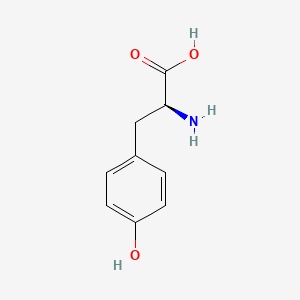 |
0.447 | ||
| ENC001091 | 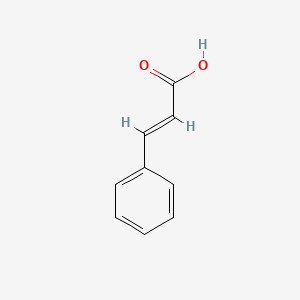 |
0.524 | D0U5QK | 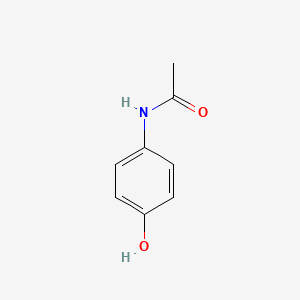 |
0.432 | ||
| ENC001101 | 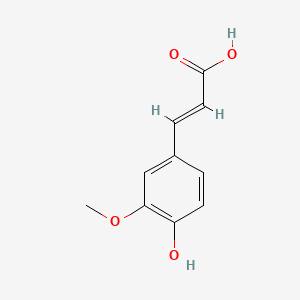 |
0.511 | D0B3QM | 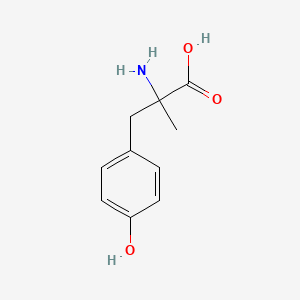 |
0.429 | ||
| ENC001547 | 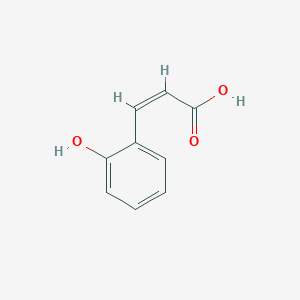 |
0.500 | D02WAB | 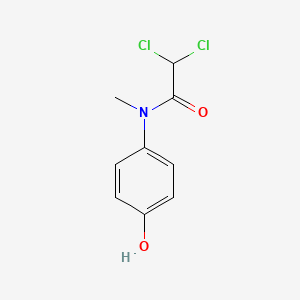 |
0.373 | ||
| ENC000006 | 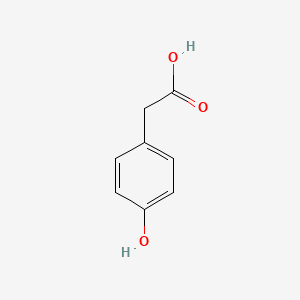 |
0.500 | D07HBX |  |
0.333 | ||
| ENC001676 | 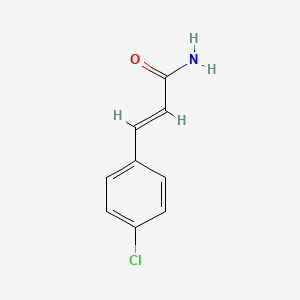 |
0.500 | D0W1RY | 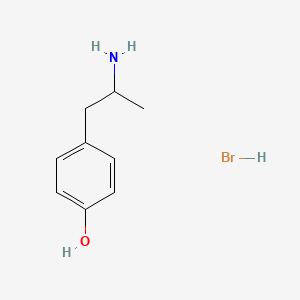 |
0.333 | ||