NPs Basic Information
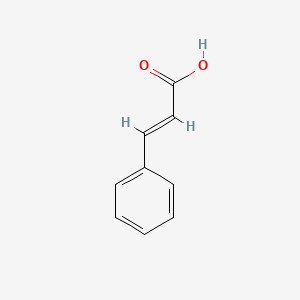
|
Name |
Cinnamic acid
|
| Molecular Formula | C9H8O2 | |
| IUPAC Name* |
(E)-3-phenylprop-2-enoic acid
|
|
| SMILES |
C1=CC=C(C=C1)/C=C/C(=O)O
|
|
| InChI |
InChI=1S/C9H8O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H,(H,10,11)/b7-6+
|
|
| InChIKey |
WBYWAXJHAXSJNI-VOTSOKGWSA-N
|
|
| Synonyms |
CINNAMIC ACID; TRANS-CINNAMIC ACID; 140-10-3; 621-82-9; 3-Phenylacrylic acid; (E)-Cinnamic acid; trans-3-Phenylacrylic acid; E-Cinnamic Acid; Phenylacrylic acid; (E)-3-phenylprop-2-enoic acid; Zimtsaeure; (2E)-3-phenylprop-2-enoic acid; trans-Cinnamate; 3-Phenylpropenoic acid; Cinnamic acid, (E)-; trans-beta-Carboxystyrene; 3-phenylprop-2-enoic acid; 2-Propenoic acid, 3-phenyl-, (2E)-; Cinnamylic acid; (E)-cinnamate; Benzeneacrylic acid; (E)-3-Phenyl-2-propenoic acid; trans-3-Phenyl-2-propenoic acid; 3-Phenyl-2-propenoic acid; (2E)-3-Phenyl-2-propenoic acid; 2-Propenoic acid, 3-phenyl-, (E)-; t-Cinnamic acid; Benzenepropenoic acid; MFCD00004369; beta-Phenylacrylic acid; PHENYLETHYLENECARBOXYLIC ACID; Cinnamic acid(only trans); Benzylideneacetic acid; (2E)-3-phenylacrylic acid; CHEMBL27246; CHEBI:35697; (2E)-2-Phenyl-2-propenoic acid; U14A832J8D; NSC-9189; FEMA No. 2288; NSC-44010; NSC-623441; NCGC00165979-01; DSSTox_CID_2489; DSSTox_RID_76603; DSSTox_GSID_22489; 28934-71-6; CAS-140-10-3; CCRIS 3190; .beta.-Phenylacrylic acid; EINECS 205-398-1; NSC 44010; (E)-3-Phenylacrylic acid; BRN 1905952; UNII-U14A832J8D; AI3-23709; trans-Zimtsaeure; trans cinnamic acid; 5-Thiazolamine?HCl; b-Phenylacrylic acid; Cinnamic acid, E-; Cinnamic Acid Natural; CINNAMIC ACIDUM; trans-b-Carboxystyrene; Cinnamicacid(onlytrans); PhCH=CHCO2H; trans-3-Phenylacrylate; (E)-3-Phenylacrylate; E-3-phenylpropenoic acid; Trans-Cinnamic Acid,(S); bmse000124; CINNAMIC ACID [MI]; SCHEMBL1332; trans-.beta.-Carboxystyrene; trans-Cinnamic acid, 97%; trans-Cinnamic acid, 99%; WLN: QV1U1R; (E)-3-phenyl-acrylic acid; 3-phenyl-2E-propenoic acid; CINNAMIC ACID [FCC]; Zimtsaeure | trans-Cinnamate; (E)-3-phenylprop-2-enoate; CINNAMIC ACID [FHFI]; CINNAMIC ACID [INCI]; trans-3-Phenyl-2-propenoate; 4-09-00-02002 (Beilstein Handbook Reference); BIDD:ER0586; CINNAMIC ACID [MART.]; tert-.beta.-Phenylacrylic acid; trans-Cinnamic acid, >=99%; (2E)-2-Phenyl-2-propenoate; (2E)-3-Phenyl-2-propenoate; GTPL3203; CINNAMIC ACID [USP-RS]; CINNAMIC ACID [WHO-DD]; DTXSID5022489; BDBM16430; CHEBI:27386; HY-N0610A; NSC9189; NSC44010; STR00363; trans-Cinnamic acid, >=99%, FG; Tox21_112279; Tox21_302137; BBL036895; NSC623441; s3677; STK286093; ZINC16051516; AKOS000118871; CCG-214473; CS-W020005; trans-Cinnamic acid, analytical standard; NCGC00165979-04; NCGC00165979-06; NCGC00255114-01; AC-34658; AS-75479; BP-20203; DB-003797; EN300-19599; trans-Cinnamic acid, purum, >=99.0% (T); A14569; C00423; D70605; EN300-306004; AB00374254-03; trans-cinnamic acid (trans-3-phenylacrylic acid); trans-Cinnamic Acid [Matrix for MALDI-TOF/MS]; A833631; Q164785; SR-05000002380; trans-Cinnamic acid, natural, >=99%, FCC, FG; Q-100150; SR-05000002380-1; W-105037; F2191-0134; trans-Cinnamic Acid Zone Refined (number of passes:40); trans-Cinnamic acid; Phenylacrylic acid;Cinnamylic acid; Z104474406; 1BE36587-A165-4142-9340-18FFE3E03426; CINNAMIC ACID (CONSTITUENT OF CINNAMOMUM VERUM BARK) [DSC]; Cinnamic acid, United States Pharmacopeia (USP) Reference Standard; TRANS-CINNAMIC ACID (CONSTITUENT OF CINNAMOMUM CASSIA BARK) [DSC]
|
|
| CAS | 140-10-3 | |
| PubChem CID | 444539 | |
| ChEMBL ID | CHEMBL27246 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 148.16 | ALogp: | 2.1 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.654 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.657 | MDCK Permeability: | 0.00001430 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.015 |
| 30% Bioavailability (F30%): | 0.499 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.333 | Plasma Protein Binding (PPB): | 79.66% |
| Volume Distribution (VD): | 0.246 | Fu: | 9.46% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.172 | CYP1A2-substrate: | 0.069 |
| CYP2C19-inhibitor: | 0.04 | CYP2C19-substrate: | 0.055 |
| CYP2C9-inhibitor: | 0.091 | CYP2C9-substrate: | 0.754 |
| CYP2D6-inhibitor: | 0.048 | CYP2D6-substrate: | 0.198 |
| CYP3A4-inhibitor: | 0.013 | CYP3A4-substrate: | 0.071 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.795 | Half-life (T1/2): | 0.855 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.757 |
| Drug-inuced Liver Injury (DILI): | 0.921 | AMES Toxicity: | 0.019 |
| Rat Oral Acute Toxicity: | 0.018 | Maximum Recommended Daily Dose: | 0.011 |
| Skin Sensitization: | 0.929 | Carcinogencity: | 0.091 |
| Eye Corrosion: | 0.932 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.241 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001443 | 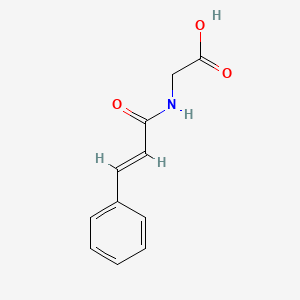 |
0.622 | D01ZJK | 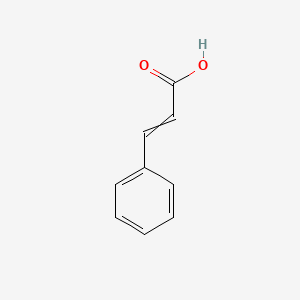 |
1.000 | ||
| ENC001615 | 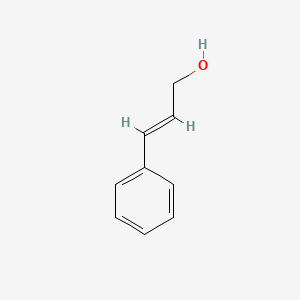 |
0.579 | D0X9RY | 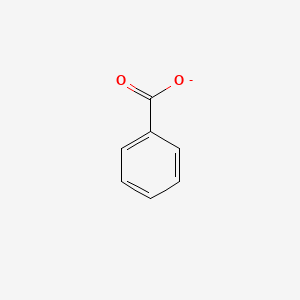 |
0.436 | ||
| ENC000023 | 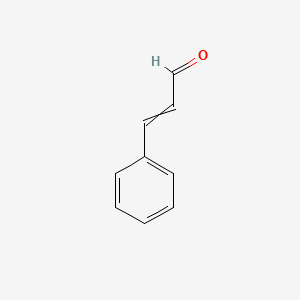 |
0.579 | D0V9EN | 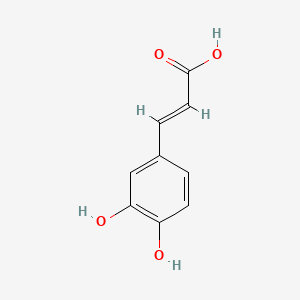 |
0.435 | ||
| ENC001547 | 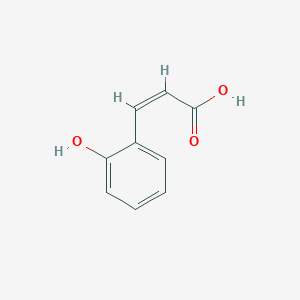 |
0.561 | D0R1CR | 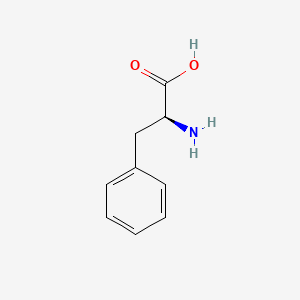 |
0.422 | ||
| ENC001616 | 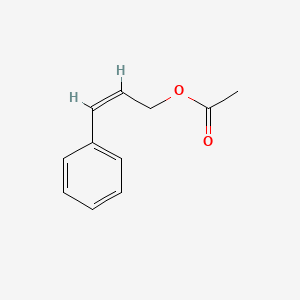 |
0.545 | D0C7AA | 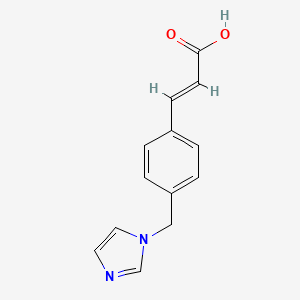 |
0.404 | ||
| ENC000012 | 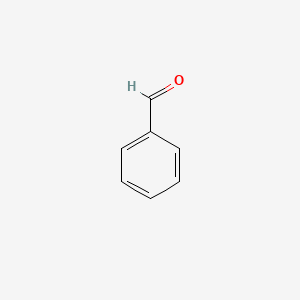 |
0.543 | D05OIS | 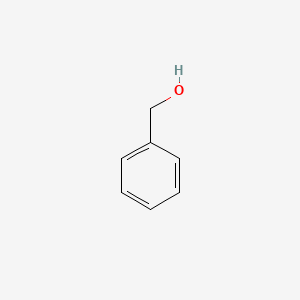 |
0.385 | ||
| ENC001420 | 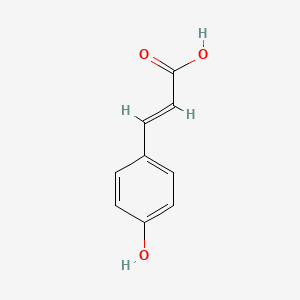 |
0.524 | D07HBX |  |
0.381 | ||
| ENC000013 | 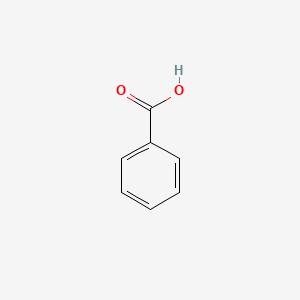 |
0.514 | D0L1WV | 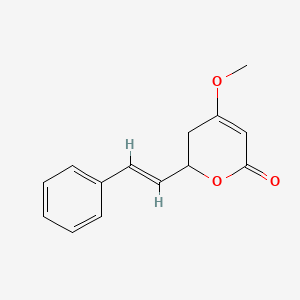 |
0.379 | ||
| ENC001736 | 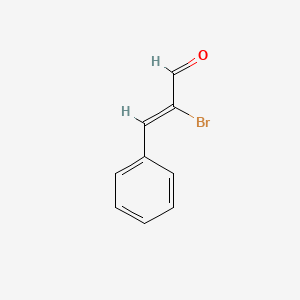 |
0.512 | D03KOZ | 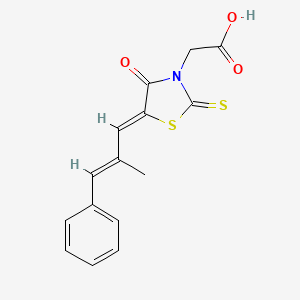 |
0.369 | ||
| ENC001428 | 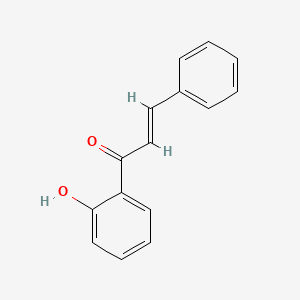 |
0.509 | D0P2GK | 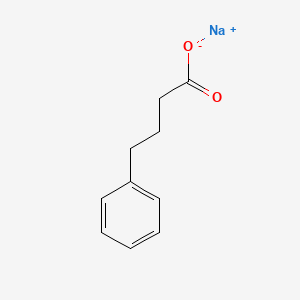 |
0.347 | ||