NPs Basic Information
|
Name |
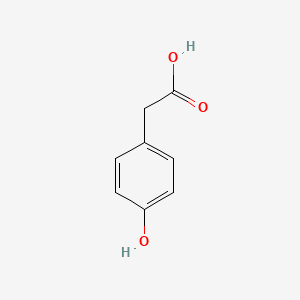
4-Hydroxyphenylacetic acid
|
| Molecular Formula | C8H8O3 | |
| IUPAC Name* |
2-(4-hydroxyphenyl)acetic acid
|
|
| SMILES |
C1=CC(=CC=C1CC(=O)O)O
|
|
| InChI |
InChI=1S/C8H8O3/c9-7-3-1-6(2-4-7)5-8(10)11/h1-4,9H,5H2,(H,10,11)
|
|
| InChIKey |
XQXPVVBIMDBYFF-UHFFFAOYSA-N
|
|
| Synonyms |
4-hydroxyphenylacetic acid; 156-38-7; 2-(4-hydroxyphenyl)acetic acid; p-hydroxyphenylacetic acid; (4-Hydroxyphenyl)acetic acid; benzeneacetic acid, 4-hydroxy-; 4-Hydroxybenzeneacetic acid; 4-hydroxyphenylacetate; (p-Hydroxyphenyl)acetic acid; Parahydroxy phenylacetic acid; Acetic acid, (p-hydroxyphenyl)-; 4-Carboxymethylphenol; MFCD00004347; 4-Hydroxyphenyl acetic acid; p-hydroxyphenylacetate; Parahydroxy phenylacetate; 3J9SHG0RCN; p-Hydroxyphenyl acetic acid; 4-hydroxyphenyl-acetic acid; CHEMBL1772; 4-hydroxy phenyl acetic acid; (4-hydroxy-phenyl)-acetic acid; CHEBI:18101; NSC-25066; NSC-27460; 4-hydroxybenzeneacetate; 4HP; (p-hydroxyphenyl)acetate; 2-[4-(hydroxy)phenyl)acetic acid; 4-HPA; 2-(4-(HYDROXY)PHENYL)ACETIC ACID; 4-(Carboxymethyl)phenol; EINECS 205-851-3; UNII-3J9SHG0RCN; NSC 25066; BRN 1448766; AI3-17755; 3pcg; Parahydroxyphenylacetate; 1ai6; 4-Hydroxy-Benzeneacetate; 4-hydroxyphenyacetic acid; 4-hydroxyphenylactic acid; 4-hyroxyphenylacetic acid; (p-hydroxyphenyl)-Acetate; ChemDiv3_005483; bmse000455; 4- hydroxyphenylacetic acid; 4-hydroxy-phenylacetic acid; 4-hydroxyphenylethanoic acid; (4-hydroxy-phenyl)-acetate; p-hydroxy phenyl acetic acid; 4-Hydroxy-Benzeneacetic acid; 4-Hydroxybenzene acetic acid; SCHEMBL75700; 4-hydroxy-phenyl acetic acid; 4-10-00-00543 (Beilstein Handbook Reference); MLS001066398; (p-hydroxyphenyl)-Acetic acid; (4-hydroxyphenyl) acetic acid; (4-hydroxyphenyl)-acetic acid; (4-hydroxyphenyl)ethanoic acid; Acetic acid, 4-hydroxyphenyl-; (4-Hydroxy-phenyl)-essigsaeure; 4-Hydroxyphenylacetic Acid,(S); DTXSID5059745; 4-Hydroxyphenylacetic acid, 98%; HMS1488J05; HMS2760I07; ZINC213065; CS-D1503; HY-N1902; NSC25066; NSC27460; AC7824; BBL027456; BDBM50339586; s4863; STL377918; AKOS000277614; AM84511; CCG-266227; PS-5568; IDI1_023393; AC-10104; SMR000020068; SY004128; DB-043314; EU-0016214; FT-0618732; H0290; EN300-18714; C00642; H-7100; A809742; AM-814/41090691; Q4637160; 4-Hydroxyphenylacetic acid, Vetec(TM) reagent grade, 98%; B977C251-72C6-4D13-AD85-937DCA3E6AF2
|
|
| CAS | 156-38-7 | |
| PubChem CID | 127 | |
| ChEMBL ID | CHEMBL1772 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.15 | ALogp: | 0.8 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.674 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.105 | MDCK Permeability: | 0.00002550 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.024 |
| 30% Bioavailability (F30%): | 0.007 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.104 | Plasma Protein Binding (PPB): | 57.83% |
| Volume Distribution (VD): | 0.237 | Fu: | 36.94% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.028 | CYP1A2-substrate: | 0.076 |
| CYP2C19-inhibitor: | 0.035 | CYP2C19-substrate: | 0.065 |
| CYP2C9-inhibitor: | 0.03 | CYP2C9-substrate: | 0.96 |
| CYP2D6-inhibitor: | 0.01 | CYP2D6-substrate: | 0.393 |
| CYP3A4-inhibitor: | 0.014 | CYP3A4-substrate: | 0.116 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.961 | Half-life (T1/2): | 0.916 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.102 |
| Drug-inuced Liver Injury (DILI): | 0.831 | AMES Toxicity: | 0.09 |
| Rat Oral Acute Toxicity: | 0.448 | Maximum Recommended Daily Dose: | 0.007 |
| Skin Sensitization: | 0.374 | Carcinogencity: | 0.382 |
| Eye Corrosion: | 0.922 | Eye Irritation: | 0.984 |
| Respiratory Toxicity: | 0.044 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000774 | 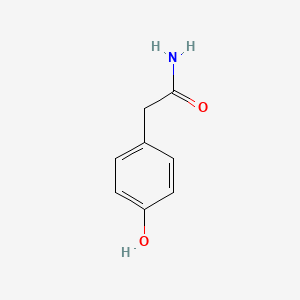 |
0.714 | D01CRB | 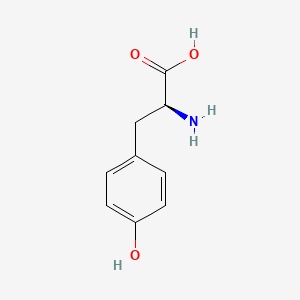 |
0.625 | ||
| ENC004860 | 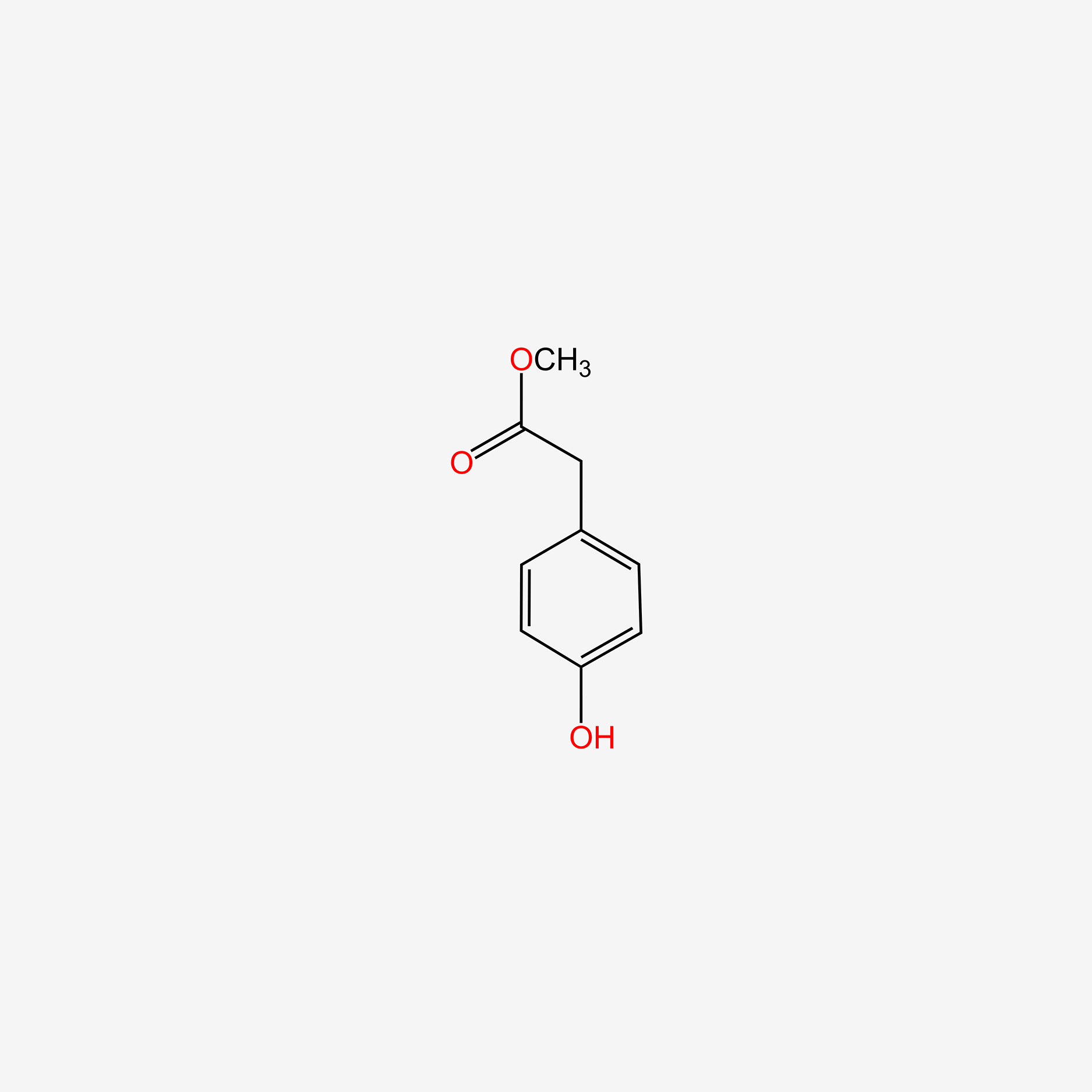 |
0.658 | D0B3QM | 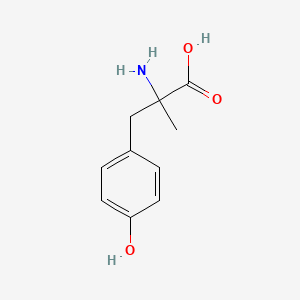 |
0.595 | ||
| ENC000129 | 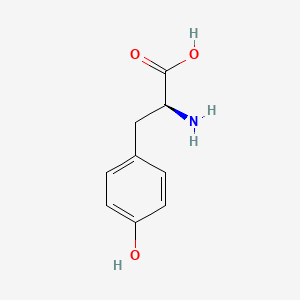 |
0.625 | D02AQY | 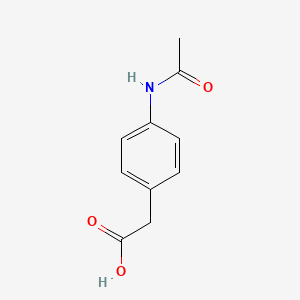 |
0.545 | ||
| ENC000007 | 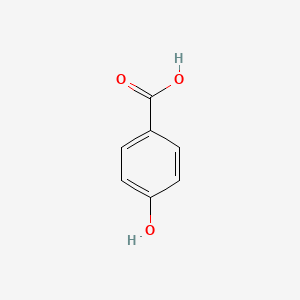 |
0.583 | D0W1RY | 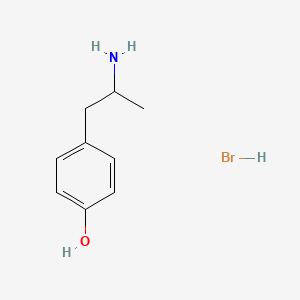 |
0.488 | ||
| ENC000350 | 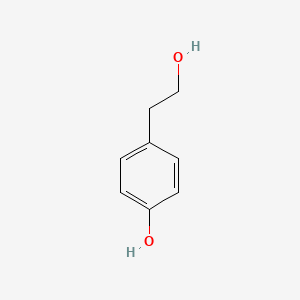 |
0.568 | D03UOT |  |
0.486 | ||
| ENC000870 | 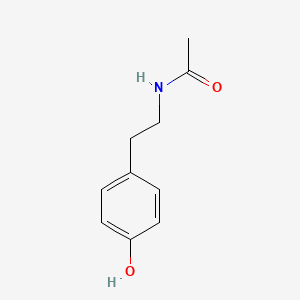 |
0.535 | D0U5QK | 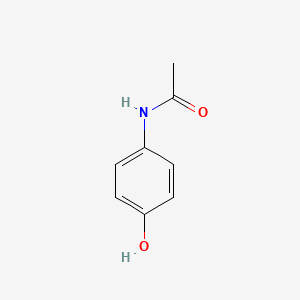 |
0.463 | ||
| ENC001422 | 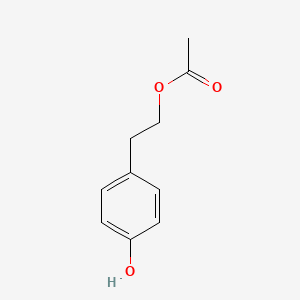 |
0.535 | D0Y7EM | 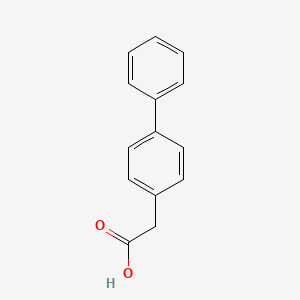 |
0.462 | ||
| ENC000676 | 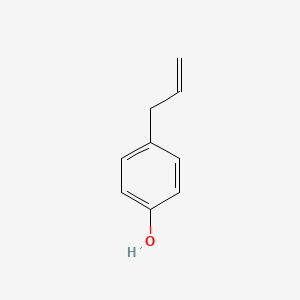 |
0.526 | D0S2BV | 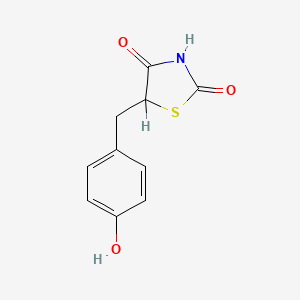 |
0.412 | ||
| ENC000740 | 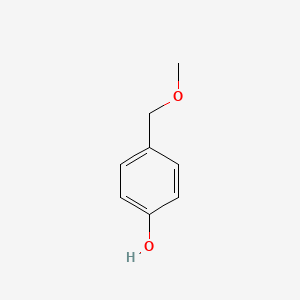 |
0.526 | D02WAB | 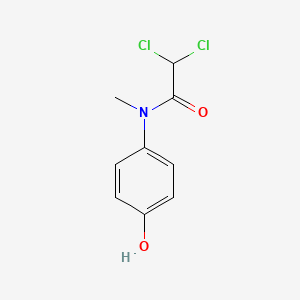 |
0.396 | ||
| ENC000862 | 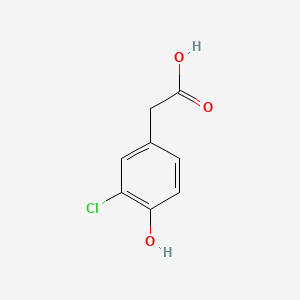 |
0.512 | D0R1QE | 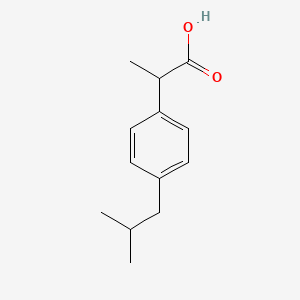 |
0.373 | ||