NPs Basic Information
|
Name |
4-Methoxycinnamic acid
|
| Molecular Formula | C10H10O3 | |
| IUPAC Name* |
(E)-3-(4-methoxyphenyl)prop-2-enoic acid
|
|
| SMILES |
COC1=CC=C(C=C1)/C=C/C(=O)O
|
|
| InChI |
InChI=1S/C10H10O3/c1-13-9-5-2-8(3-6-9)4-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-4+
|
|
| InChIKey |
AFDXODALSZRGIH-QPJJXVBHSA-N
|
|
| Synonyms |
4-Methoxycinnamic acid; 830-09-1; 943-89-5; P-METHOXYCINNAMIC ACID; 3-(4-methoxyphenyl)acrylic acid; trans-4-Methoxycinnamic acid; (E)-3-(4-Methoxyphenyl)acrylic acid; (E)-3-(4-methoxyphenyl)prop-2-enoic acid; para-methoxycinnamic acid; (E)-4-METHOXYCINNAMIC ACID; 2-Propenoic acid, 3-(4-methoxyphenyl)-; Cinnamic acid, p-methoxy-; 3-(4-Methoxyphenyl)-2-propenoic acid; 4-Methoxycinnamate; (E)-3-(4-methoxyphenyl)-2-propenoic acid; O-Methyl-p-coumaric acid; 2-Propenoic acid, 3-(4-methoxyphenyl)-, (2E)-; (2E)-3-(4-methoxyphenyl)prop-2-enoic acid; Cinnamic acid, 4-methoxy-; 4-Methoxycinnamicacid; Bernel hydro; METHOXYCINNAMIC ACID, PARA; (E)-p-Methoxycinnamic acid; MFCD00004398; p-methoxycinnamate; 4-Methoxycinnamic acid, predominantly trans; NSC-5303; NSC-623437; CHEMBL95770; 4-Methoxy-(2E)-cinnamic acid; (2E)-3-(4-METHOXYPHENYL)ACRYLIC ACID; trans-2-Propenoic acid, 3-(4-methoxyphenyl)-; 6G4ML8401A; NCGC00159448-02; (E)-3-(4-Methoxy-phenyl)-acrylic acid; 3-(4-methoxyphenyl)prop-2-enoic acid; 30-09-1; 4-Methoxy cinnamic acid; PMCA; NSC 5303; P-Methoxy ciannamic acid; EINECS 212-594-0; UNII-6G4ML8401A; AI3-23399; K3Z; EINECS 213-405-4; para-methoxycinnamate; 4-Methoxy cinnamate; P-Methoxy ciannamate; O-Methyl-p-coumarate; p-MCA; trans-4-Methoxycinnamate; O-Methyl-p-cumaric Acid; bmse010212; 4-Methoxybenzeneacrylic acid; DSSTox_CID_26059; DSSTox_RID_81310; trans-4-methoxycinnamic-acid; 2-Propenoic acid, 3-(4-methoxyphenyl)-, (E)-; DSSTox_GSID_46059; SCHEMBL58699; (E)-p-Methoxy-cinnamic acid; MLS002473129; trans-4-methoxy-cinnamic acid; 3-(4-Methoxy-phenyl)-acrylate; DTXSID1046059; AFDXODALSZRGIH-QPJJXVBHSA-; CHEBI:48541; ZINC77999; AMY4119; NSC5303; CHEBI:143736; HMS1783A08; HMS2267B19; (E)-3-(4-Methoxyphenyl)acrylate; 3-(4-methoxyphenyl)-2-Propenoate; ALBB-011726; BCP21420; HY-N1387; STR01310; Tox21_111674; BBL012085; BDBM50146453; NSC623437; STK005095; AKOS000118882; PS-5777; (E)-3-(4-methoxyphenyl)-acrylic acid; NCGC00159448-03; NCGC00159448-04; AC-10371; CAS-943-89-5; SMR000112200; 4-Methoxycinnamic acid, >=98.0% (GC); p-Methoxycinnamic acid, predominantly trans; CS-0016807; M0576; EN300-312690; (2E)-3-(4-Methoxyphenyl)-2-propenoic acid #; 4-Methoxycinnamic acid, predominantly trans, 99%; 830M091; A837404; A840486; AE-641/00135031; TRANS-3-(4-METHOXYPHENYL)-2-PROPENOIC ACID; W-100196; W-104154; Q63391599; Z56931888; 4-Methoxycinnamic acid;3-(4-Methoxyphenyl)acrylic acid; F1638-0067; 0C546A89-9721-4B4E-89EE-7EC28A9A3391
|
|
| CAS | 943-89-5 | |
| PubChem CID | 699414 | |
| ChEMBL ID | CHEMBL95770 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 178.18 | ALogp: | 1.8 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.723 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.624 | MDCK Permeability: | 0.00001120 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.042 |
| Human Intestinal Absorption (HIA): | 0.01 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.898 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.312 | Plasma Protein Binding (PPB): | 80.83% |
| Volume Distribution (VD): | 0.221 | Fu: | 8.53% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.211 | CYP1A2-substrate: | 0.225 |
| CYP2C19-inhibitor: | 0.071 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.064 | CYP2C9-substrate: | 0.872 |
| CYP2D6-inhibitor: | 0.113 | CYP2D6-substrate: | 0.477 |
| CYP3A4-inhibitor: | 0.032 | CYP3A4-substrate: | 0.074 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.341 | Half-life (T1/2): | 0.835 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.286 |
| Drug-inuced Liver Injury (DILI): | 0.934 | AMES Toxicity: | 0.062 |
| Rat Oral Acute Toxicity: | 0.026 | Maximum Recommended Daily Dose: | 0.018 |
| Skin Sensitization: | 0.935 | Carcinogencity: | 0.667 |
| Eye Corrosion: | 0.807 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.298 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
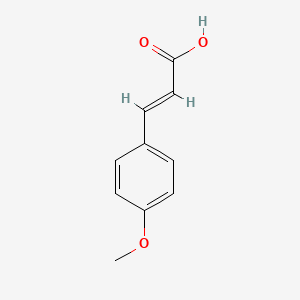
| ENC001578 |  |
0.660 | D01ZJK |  |
0.489 | ||
| ENC001420 | 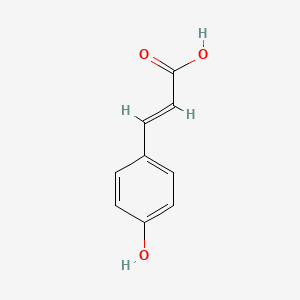 |
0.643 | D0C7AA |  |
0.466 | ||
| ENC001460 | 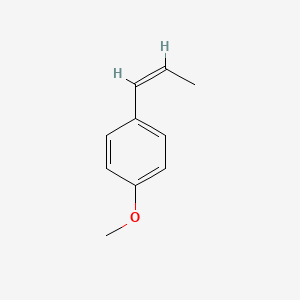 |
0.634 | D0V9EN |  |
0.449 | ||
| ENC001101 | 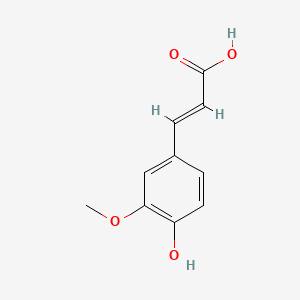 |
0.542 | D02DPU | 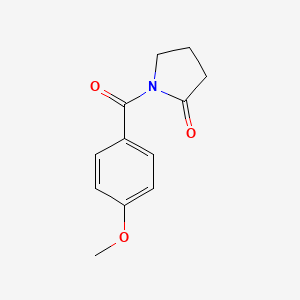 |
0.373 | ||
| ENC000201 | 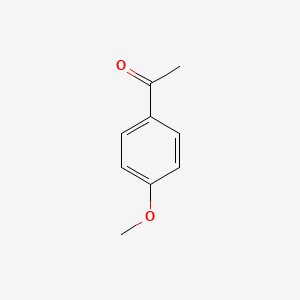 |
0.500 | D0DJ1B |  |
0.361 | ||
| ENC001091 | 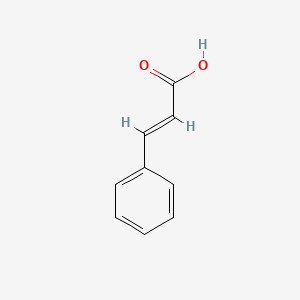 |
0.489 | D0E6OC | 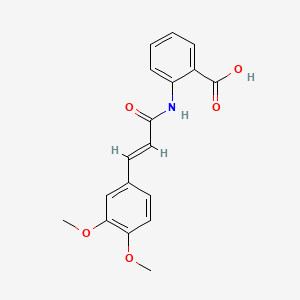 |
0.338 | ||
| ENC000318 | 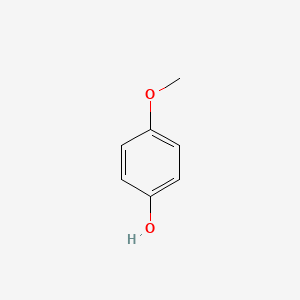 |
0.488 | D01NJI | 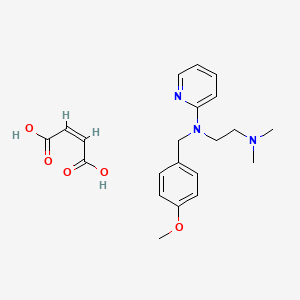 |
0.333 | ||
| ENC001676 | 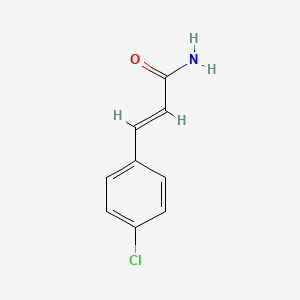 |
0.468 | D0E9CD |  |
0.320 | ||
| ENC000298 | 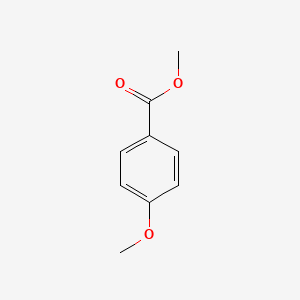 |
0.468 | D0EJ6O | 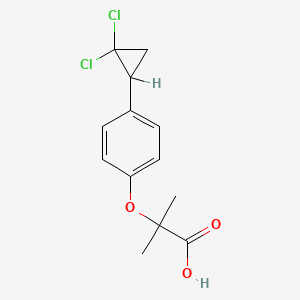 |
0.317 | ||
| ENC001456 | 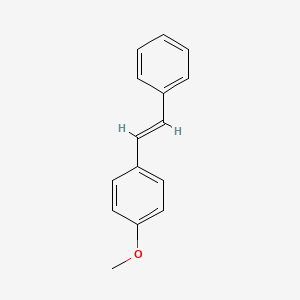 |
0.456 | D05CKR | 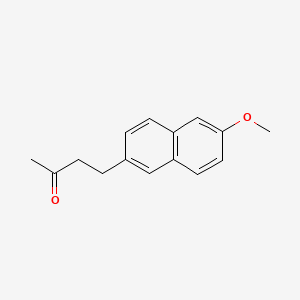 |
0.313 | ||