NPs Basic Information
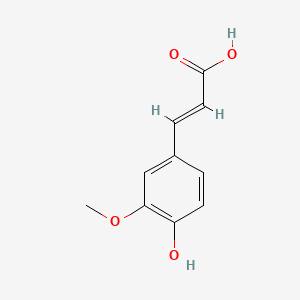
|
Name |
Ferulic acid
|
| Molecular Formula | C10H10O4 | |
| IUPAC Name* |
(E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid
|
|
| SMILES |
COC1=C(C=CC(=C1)/C=C/C(=O)O)O
|
|
| InChI |
InChI=1S/C10H10O4/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+
|
|
| InChIKey |
KSEBMYQBYZTDHS-HWKANZROSA-N
|
|
| Synonyms |
ferulic acid; trans-Ferulic Acid; 1135-24-6; 537-98-4; 4-Hydroxy-3-methoxycinnamic acid; trans-4-Hydroxy-3-methoxycinnamic acid; 3-(4-Hydroxy-3-methoxyphenyl)acrylic acid; (E)-Ferulic acid; ferulate; Coniferic acid; 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-; 3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid; Ferulic acid, trans-; Fumalic acid; (E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid; (2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid; Cinnamic acid, 4-hydroxy-3-methoxy-; 3-methoxy-4-hydroxycinnamic acid; Cinnamic acid, 4-hydroxy-3-methoxy-, (E)-; MFCD00004400; (E)-4-Hydroxy-3-methoxycinnamic acid; 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-, (2E)-; (E)-4'-Hydroxy-3'-methoxycinnamic acid; (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid; (2E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid; Cinnamic acid, 4-hydroxy-3-methoxy-, trans-; 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-, (E)-; AVM951ZWST; (E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid; 97274-61-8; (2E)-3-(4-Hydroxy-3-methoxyphenyl)acrylic acid; Fumalic acid (Ferulic acid); 4-Hydroxy-3-methoxycinnamate; CHEMBL32749; CHEBI:17620; NSC2821; NSC-2821; 3-Methoxy-4-hydroxy-trans-cinnamate; NSC-51986; (E)-3-(4-hydroxy-3-methoxy-phenyl)prop-2-enoic acid; (E)-Ferulate; 3-(4-Hydroxy-3-methoxyphenyl)propenoic acid; trans-Ferulic Acid (purified by sublimation); CINNAMIC ACID,4-HYDROXY,3-METHOXY FERULIC ACID; caffeic acid 3-methyl ether; 3-methoxy-4-hydroxy-trans-cinnamic acid; SMR000112202; 4-Hydroxy-3-methoxy cinnamic acid; EINECS 208-679-7; UNII-AVM951ZWST; ferulic acid (trans-4-hydroxy-3-methoxycinnamic acid); ferulasaure; Ferulicacid; trans-Ferulate; (E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoate; CCRIS 3256; CCRIS 7127; CCRIS 7575; HSDB 7663; trans-FerulicAcid; NSC-674320; NSC 2821; Ferulic acid, E-; EINECS 214-490-0; NSC 51986; (E)-Coniferic acid; trans-4-Hydroxy-3-methoxycinnamicacid; Ferulic acid (M5); NSC 674320; Ferulic Acid ,(S); FERULIC-ACID; Ferulic Acid, Synthetic; Spectrum5_000554; bmse000459; bmse000587; bmse010211; FERULIC ACID [MI]; trans-Ferulic acid, 99%; FERULIC ACID [HSDB]; FERULIC ACID [INCI]; SCHEMBL15673; BSPBio_003168; MLS001066385; MLS001332483; MLS001332484; MLS002207079; MLS006011435; SPECTRUM1501017; trans-Ferulic acid, >=99%; FERULIC ACID [USP-RS]; FERULIC ACID [WHO-DD]; ZINC58258; DTXSID70892035; HMS1921D05; HMS2269P04; (E)-4-Hydroxy-3-methoxycinnamate; trans-4-Hydroxy-3-methoxycinnamate; ALBB-013505; BCP21231; BCP21789; HY-N0060; NSC51986; STR00961; (E)-4-hydroxy-3-methoxy-Cinnamate; TRANS-FERULIC ACID [WHO-DD]; (E)4-hydroxy-3-methoxycinnamic acid; AC7905; BBL010345; BDBM50214744; CCG-38860; s2300; STK801551; AKOS000263735; AC-7965; BCP9000163; DB07767; PS-3435; SDCCGMLS-0066667.P001; trans-3-methoxy-4-hydroxycinnamic acid; (E)-4-hydroxy-3-methoxy-Cinnamic acid; 3-(4-Hydroxy-3-methoxyphenyl)propenoate; 4-Hydroxy-3-methoxycinnamic acid, trans; NCGC00094889-01; NCGC00094889-02; NCGC00094889-03; NCGC00094889-04; AC-10321; BS-17543; SMR004703246; AM20060784; CS-0007108; F1257; H0267; SW219616-1; EN300-16798; C01494; Trans-3-(4-hydroxy-3-methoxyphenyl)acrylic acid; A829775; Q417362; SR-01000765539; (2E)-3-(4-Hydroxy-3-methoxyphenyl)-2-propenoate; (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoicacid; J-002980; SR-01000765539-3; Z56782558; (E)-3-(3-methoxy-4-oxidanyl-phenyl)prop-2-enoic acid; 3-(4-HYDROXY-3-METHOXYPHENYL)PROP-2-ENOICACID; FERULIC ACID (CONSTITUENT OF BLACK COHOSH) [DSC]; 055E203F-B305-4B7F-8CE7-F9C0C03AB609; 3986A1BE-A670-4B06-833B-E17253079FD8; Ferulic acid, European Pharmacopoeia (EP) Reference Standard; trans-Ferulic acid, certified reference material, TraceCERT(R); Diethyl2-(acetamido)-2-(2-(bromomethyl)-5-nitrobenzyl)malonate; Ferulic acid, United States Pharmacopeia (USP) Reference Standard; trans-Ferulic acid, matrix substance for MALDI-MS, >=99.0% (HPLC); Ferulic Acid, Pharmaceutical Secondary Standard; Certified Reference Material; 831-85-6
|
|
| CAS | 537-98-4 | |
| PubChem CID | 445858 | |
| ChEMBL ID | CHEMBL32749 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 194.18 | ALogp: | 1.5 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 66.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 14 | QED Weighted: | 0.722 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.902 | MDCK Permeability: | 0.00001460 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.086 |
| Human Intestinal Absorption (HIA): | 0.03 | 20% Bioavailability (F20%): | 0.047 |
| 30% Bioavailability (F30%): | 0.584 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.329 | Plasma Protein Binding (PPB): | 89.75% |
| Volume Distribution (VD): | 0.339 | Fu: | 6.39% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.059 | CYP1A2-substrate: | 0.478 |
| CYP2C19-inhibitor: | 0.042 | CYP2C19-substrate: | 0.057 |
| CYP2C9-inhibitor: | 0.142 | CYP2C9-substrate: | 0.367 |
| CYP2D6-inhibitor: | 0.02 | CYP2D6-substrate: | 0.199 |
| CYP3A4-inhibitor: | 0.026 | CYP3A4-substrate: | 0.075 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.48 | Half-life (T1/2): | 0.926 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.023 | Human Hepatotoxicity (H-HT): | 0.345 |
| Drug-inuced Liver Injury (DILI): | 0.511 | AMES Toxicity: | 0.114 |
| Rat Oral Acute Toxicity: | 0.733 | Maximum Recommended Daily Dose: | 0.076 |
| Skin Sensitization: | 0.929 | Carcinogencity: | 0.443 |
| Eye Corrosion: | 0.515 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.72 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001440 | 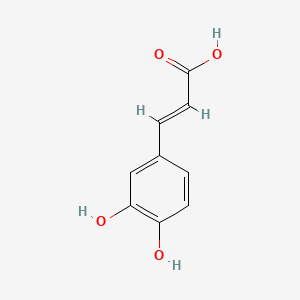 |
0.659 | D0V9EN |  |
0.659 | ||
| ENC000068 | 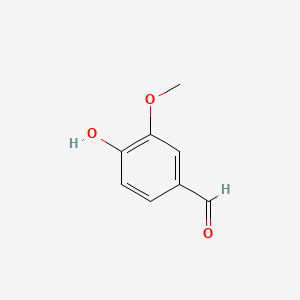 |
0.619 | D0E9CD |  |
0.478 | ||
| ENC000296 | 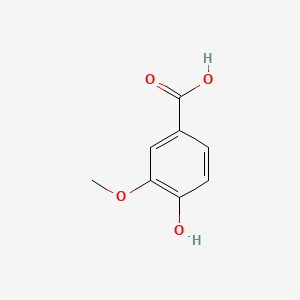 |
0.591 | D01ZJK |  |
0.408 | ||
| ENC000027 | 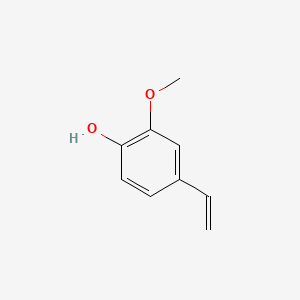 |
0.581 | D0E6OC | 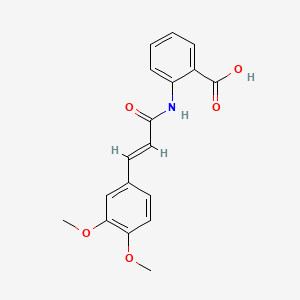 |
0.382 | ||
| ENC001848 | 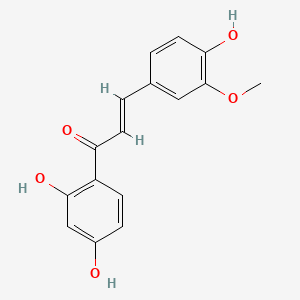 |
0.548 | D0C4YC | 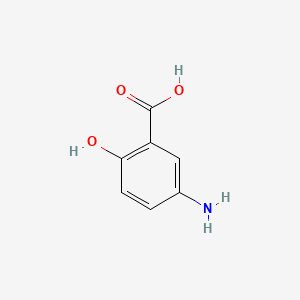 |
0.367 | ||
| ENC001441 | 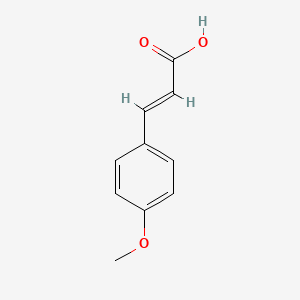 |
0.542 | D03LGG | 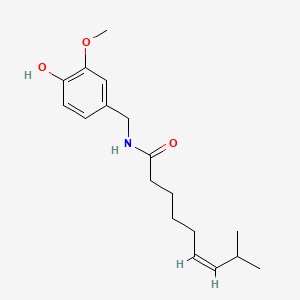 |
0.356 | ||
| ENC001056 | 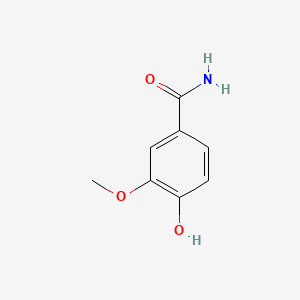 |
0.522 | D0U5CE | 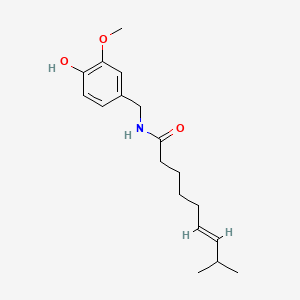 |
0.356 | ||
| ENC000777 | 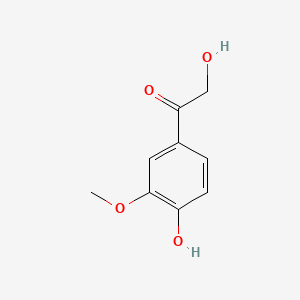 |
0.521 | D0U0OT | 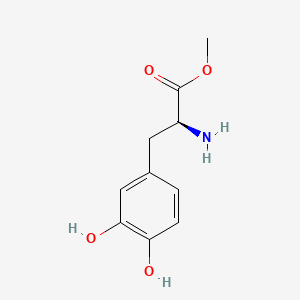 |
0.345 | ||
| ENC001420 | 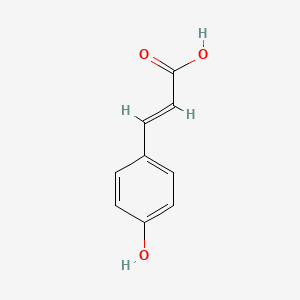 |
0.511 | D01WJL | 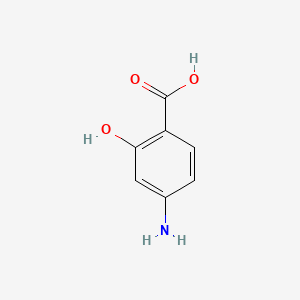 |
0.340 | ||
| ENC000325 | 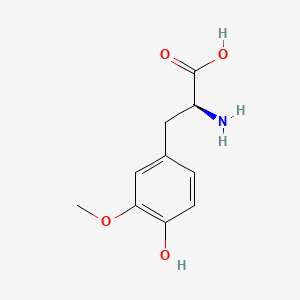 |
0.500 | D0C7AA | 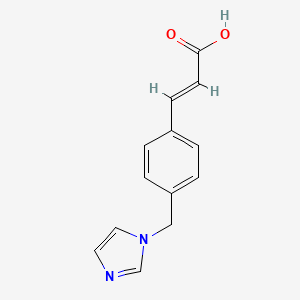 |
0.338 | ||