NPs Basic Information
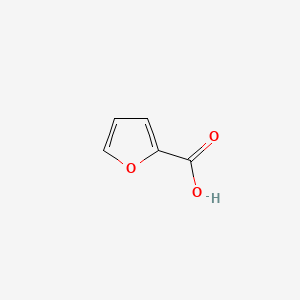
|
Name |
2-Furoic acid
|
| Molecular Formula | C5H4O3 | |
| IUPAC Name* |
furan-2-carboxylic acid
|
|
| SMILES |
C1=COC(=C1)C(=O)O
|
|
| InChI |
InChI=1S/C5H4O3/c6-5(7)4-2-1-3-8-4/h1-3H,(H,6,7)
|
|
| InChIKey |
SMNDYUVBFMFKNZ-UHFFFAOYSA-N
|
|
| Synonyms |
2-FUROIC ACID; Furan-2-carboxylic acid; 88-14-2; 2-Furancarboxylic acid; Pyromucic acid; 2-Carboxyfuran; Furancarboxylic acid; FUROIC ACID; alpha-Furoic acid; 2-furanoic acid; alpha-Furancarboxylic acid; 26447-28-9; Kyselina 2-furoova; Kyselina pyroslizova; .alpha.-Furoic acid; NSC 8842; .alpha.-Furancarboxylic acid; MFCD00003238; CHEBI:30845; NSC-8842; P577F6494A; Kyselina 2-furoova [Czech]; Kyselina pyroslizova [Czech]; CCRIS 2157; NSC-58965; EINECS 201-803-0; EINECS 247-713-5; BRN 0110149; Pyromucate; Furoate; Furoica; furanoic acid; Furancarboxylate; alpha-Furoate; furic acid; beta-Furoate; 2-Furoic acid [per EINECS]; AI3-16500; a-Furoate; b-Furoate; 2-furoicacid; a-Furoic acid; b-Furoic acid; acido 2-furoico; beta-Furoic acid; UNII-P577F6494A; 2-Furanoate; a-Furancarboxylate; acide 2-furoique; b-Furancarboxylate; Furancarbonylic acid; beta-Furancarboxylate; Furan-2-carbonsaeure; alpha-Furancarboxylate; a-Furancarboxylic acid; b-Furancarboxylic acid; Furan-2-carboxylicacid; 2-Furanecarboxylic acid; 2-furan carboxylic acid; beta-Furancarboxylic acid; furane-2-carboxylic acid; 2-Furoic acid, 98%; Furancarboxylic acid-(2); bmse000330; DSSTox_CID_21420; DSSTox_RID_79724; DSSTox_GSID_41420; SCHEMBL24446; 5-18-06-00102 (Beilstein Handbook Reference); 2-FUROIC ACID [MI]; CHEMBL1232797; DTXSID6041420; NSC8842; ZINC158555; 2-Furoic acid, analytical standard; STR01019; WGC36563; Tox21_301128; BBL009679; s4864; STK256918; 2-FUROIC ACID [USP IMPURITY]; AKOS000119036; CCG-266054; CS-W013662; HY-W012946; PS-3380; CAS-88-14-2; NCGC00248301-01; NCGC00255027-01; F0081; FT-0612470; FT-0777874; EN300-19302; C01546; AB00375923-02; AC-907/25014051; Q2210953; W-100416; F9995-1642; Z104473462
|
|
| CAS | 88-14-2 | |
| PubChem CID | 6919 | |
| ChEMBL ID | CHEMBL1232797 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 112.08 | ALogp: | 0.5 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 50.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.597 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.759 | MDCK Permeability: | 0.00001240 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.01 |
| 30% Bioavailability (F30%): | 0.109 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.312 | Plasma Protein Binding (PPB): | 80.82% |
| Volume Distribution (VD): | 0.336 | Fu: | 40.09% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.048 | CYP1A2-substrate: | 0.078 |
| CYP2C19-inhibitor: | 0.034 | CYP2C19-substrate: | 0.052 |
| CYP2C9-inhibitor: | 0.033 | CYP2C9-substrate: | 0.121 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.133 |
| CYP3A4-inhibitor: | 0.011 | CYP3A4-substrate: | 0.069 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.05 | Half-life (T1/2): | 0.911 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.061 | Human Hepatotoxicity (H-HT): | 0.255 |
| Drug-inuced Liver Injury (DILI): | 0.754 | AMES Toxicity: | 0.09 |
| Rat Oral Acute Toxicity: | 0.952 | Maximum Recommended Daily Dose: | 0.007 |
| Skin Sensitization: | 0.092 | Carcinogencity: | 0.793 |
| Eye Corrosion: | 0.621 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.942 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000480 | 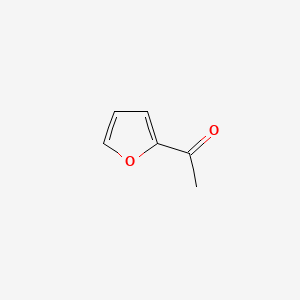 |
0.630 | D06NVJ | 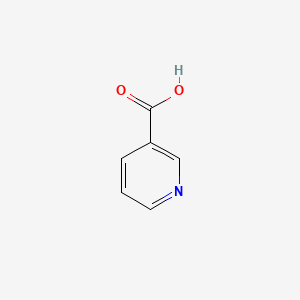 |
0.343 | ||
| ENC001133 | 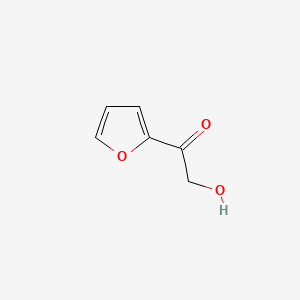 |
0.621 | D07HBX |  |
0.324 | ||
| ENC000190 | 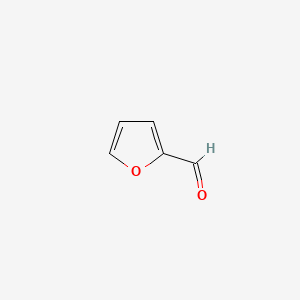 |
0.400 | D01WJL | 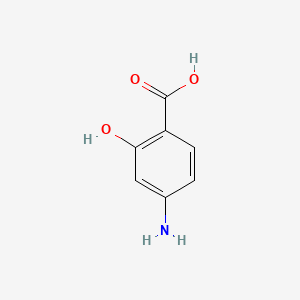 |
0.275 | ||
| ENC000189 |  |
0.400 | D0C4YC | 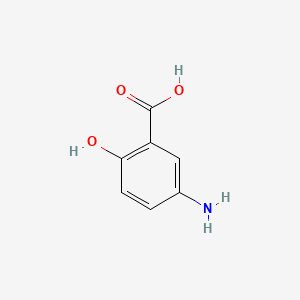 |
0.275 | ||
| ENC000439 | 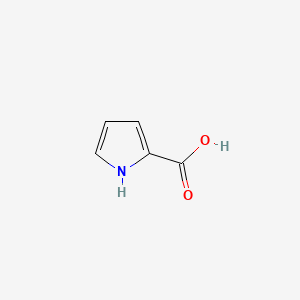 |
0.375 | D0PQ3G | 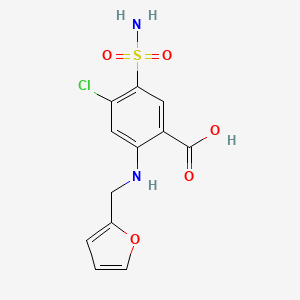 |
0.274 | ||
| ENC000748 | 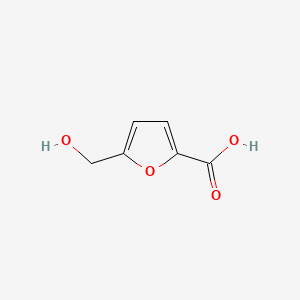 |
0.361 | D0GY5Z | 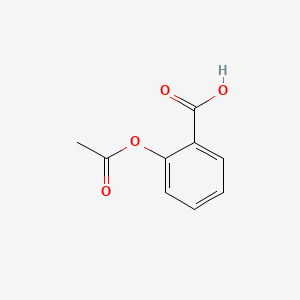 |
0.267 | ||
| ENC000678 | 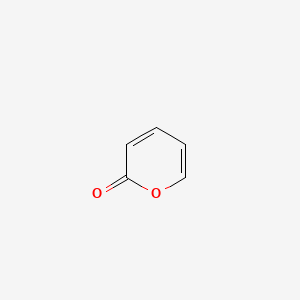 |
0.355 | D01ZJK |  |
0.262 | ||
| ENC001839 | 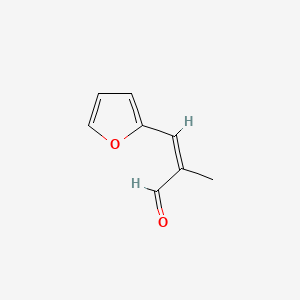 |
0.351 | D0N3UL | 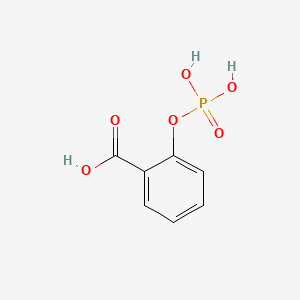 |
0.255 | ||
| ENC000056 | 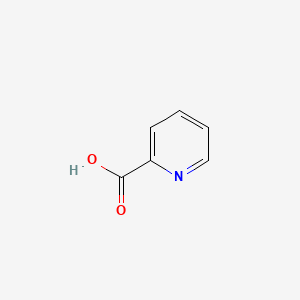 |
0.343 | D0R1CR |  |
0.250 | ||
| ENC000013 | 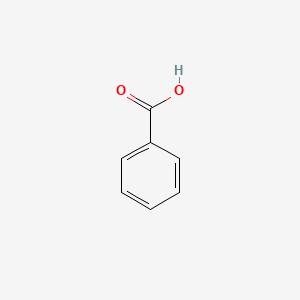 |
0.343 | D0X9RY |  |
0.237 | ||