NPs Basic Information
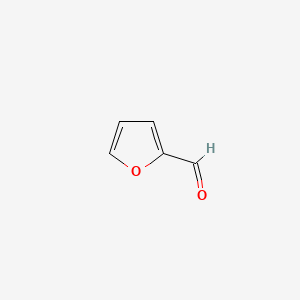
|
Name |
Furfural
|
| Molecular Formula | C5H4O2 | |
| IUPAC Name* |
furan-2-carbaldehyde
|
|
| SMILES |
C1=COC(=C1)C=O
|
|
| InChI |
InChI=1S/C5H4O2/c6-4-5-2-1-3-7-5/h1-4H
|
|
| InChIKey |
HYBBIBNJHNGZAN-UHFFFAOYSA-N
|
|
| Synonyms |
FURFURAL; 2-Furaldehyde; furan-2-carbaldehyde; 98-01-1; 2-Furancarboxaldehyde; Furaldehyde; Furfuraldehyde; Fural; 2-Formylfuran; 2-Furanaldehyde; 2-Furancarbonal; 2-Furfural; Furancarbonal; Furfurole; Furfurylaldehyde; 2-Furfuraldehyde; Pyromucic aldehyde; Furale; Furole; Furol; 2-Furylaldehyde; 2-Furylcarboxaldehyde; 2-Furyl-methanal; Furfurale; Furan-2-carboxaldehyde; Furyl-methanal; 2-Furylmethanal; 2-Furil-metanale; 2-furancarbaldehyde; Fufural; alpha-Furole; 2-Formylofuran; Nci-C56177; 2-Formyl furan; furan-2-aldehyde; 2-Furankarbaldehyd; Rcra waste number U125; alpha-Furfuraldehyde; FEMA No. 2489; Furaldehydes; .alpha.-Furole; NSC 8841; MFCD00003229; CHEBI:34768; DJ1HGI319P; NSC-8841; Furane-2-carbaldehyde; NCGC00091328-01; Quakeral; DSSTox_CID_647; DSSTox_RID_75709; DSSTox_GSID_20647; Furfural (natural); Furfurale [Italian]; Caswell No. 466; 2-Formylofuran [Polish]; 2-Furankarbaldehyd [Czech]; CAS-98-01-1; 2-Furil-metanale [Italian]; 25067-38-3; CCRIS 1044; HSDB 542; EINECS 202-627-7; UN1199; RCRA waste no. U125; UNII-DJ1HGI319P; EPA Pesticide Chemical Code 043301; BRN 0105755; Furfuralu; a-furfuraldehyde; Qo furfural; AI3-04466; a-Furole; 2-furanal; Furfural ACS grade; furan-2 carbaldehyde; Furfural, 99%; 2-furancarboxyaldehyde; 2-Furaldehyde, 8CI; 2-furan-carboxaldehyde; 2-Furanocarboxyaldehyde; FURFURAL [FHFI]; FURFURAL [HSDB]; FURFURAL [IARC]; FURFURAL [INCI]; Furfuraldehyde(Furfural); FURFURAL [FCC]; FURFURAL [MI]; 2-Furylaldehyde xypropane; WLN: T5OJ BVH; EC 202-627-7; 5-17-09-00292 (Beilstein Handbook Reference); BIDD:ER0698; Furfural, ACS reagent, 99%; CHEMBL189362; QSPL 006; QSPL 102; DTXSID1020647; FEMA 2489; Furan-2-carbaldehyde (Furfural); NSC8841; Furfural, >=98%, FCC, FG; Furfural, for synthesis, 98.0%; STR00358; ZINC3861345; Tox21_111114; Tox21_202191; Tox21_300170; BDBM50486229; Furaldehydes [UN1199] [Poison]; STL283124; AKOS000118907; AM81812; Furfural, analytical reference material; Furfural 100 microg/mL in Acetonitrile; Furfural, natural, >=98%, FCC, FG; Furfural, SAJ first grade, >=99.0%; NCGC00091328-02; NCGC00091328-03; NCGC00091328-04; NCGC00253954-01; NCGC00259740-01; BP-31002; DB-003668; CS-0015696; F0073; FT-0612462; EN300-18110; ASCORBIC ACID IMPURITY A [EP IMPURITY]; A845786; Q412429; F1294-0048; furfural; furfuraldehyde; furfurol; 2-furaldehyde; 2-furancarboxaldehyde; furan-2-carboxaldehyde
|
|
| CAS | 98-01-1 | |
| PubChem CID | 7362 | |
| ChEMBL ID | CHEMBL189362 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 96.08 | ALogp: | 0.4 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 30.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 7 | QED Weighted: | 0.496 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.361 | MDCK Permeability: | 0.00001830 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.045 |
| Human Intestinal Absorption (HIA): | 0.01 | 20% Bioavailability (F20%): | 0.008 |
| 30% Bioavailability (F30%): | 0.008 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.982 | Plasma Protein Binding (PPB): | 74.37% |
| Volume Distribution (VD): | 1.892 | Fu: | 47.93% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.748 | CYP1A2-substrate: | 0.122 |
| CYP2C19-inhibitor: | 0.122 | CYP2C19-substrate: | 0.112 |
| CYP2C9-inhibitor: | 0.022 | CYP2C9-substrate: | 0.269 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.483 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.22 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.541 | Half-life (T1/2): | 0.829 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.079 | Human Hepatotoxicity (H-HT): | 0.056 |
| Drug-inuced Liver Injury (DILI): | 0.054 | AMES Toxicity: | 0.723 |
| Rat Oral Acute Toxicity: | 0.91 | Maximum Recommended Daily Dose: | 0.024 |
| Skin Sensitization: | 0.17 | Carcinogencity: | 0.844 |
| Eye Corrosion: | 0.977 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.947 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001839 | 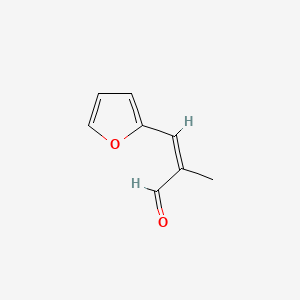 |
0.548 | D0E9CD |  |
0.220 | ||
| ENC000162 | 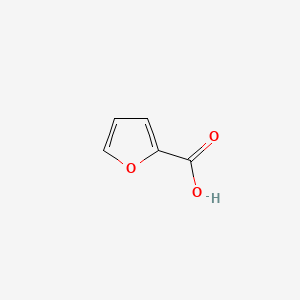 |
0.400 | D03OIW | 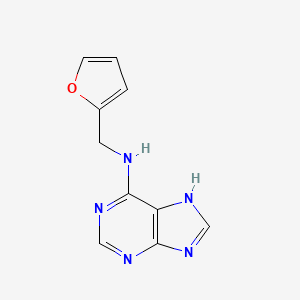 |
0.193 | ||
| ENC000480 | 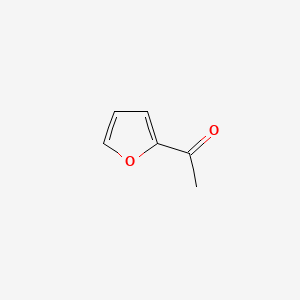 |
0.400 | D01ZJK |  |
0.186 | ||
| ENC000678 | 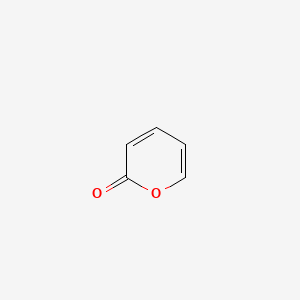 |
0.379 | D0PQ3G | 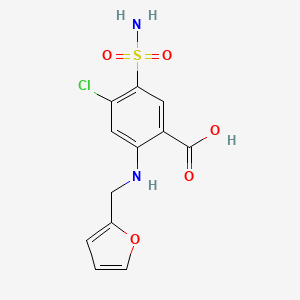 |
0.185 | ||
| ENC000189 |  |
0.379 | D0X9RY |  |
0.184 | ||
| ENC001133 | 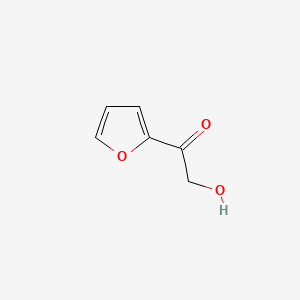 |
0.364 | D06NVJ | 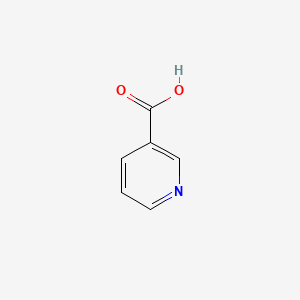 |
0.184 | ||
| ENC000412 | 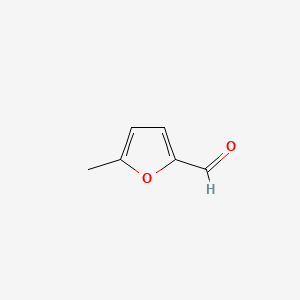 |
0.355 | D07HBX |  |
0.175 | ||
| ENC001019 | 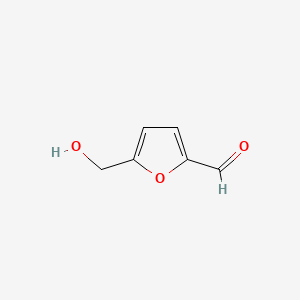 |
0.324 | D0X7NU | 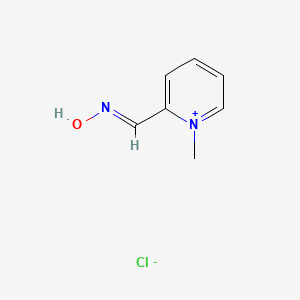 |
0.167 | ||
| ENC000546 | 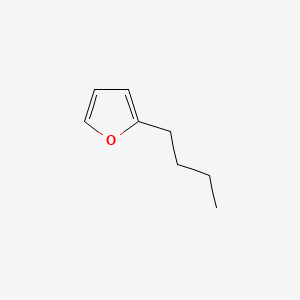 |
0.314 | D0D5GG | 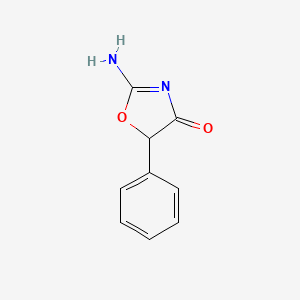 |
0.163 | ||
| ENC000479 | 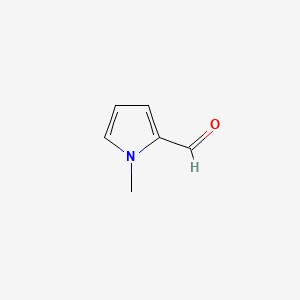 |
0.313 | D05OIS |  |
0.162 | ||