NPs Basic Information
|
Name |
5-Hydroxymethyl-2-furancarboxylic acid
|
| Molecular Formula | C6H6O4 | |
| IUPAC Name* |
5-(hydroxymethyl)furan-2-carboxylic acid
|
|
| SMILES |
C1=C(OC(=C1)C(=O)O)CO
|
|
| InChI |
InChI=1S/C6H6O4/c7-3-4-1-2-5(10-4)6(8)9/h1-2,7H,3H2,(H,8,9)
|
|
| InChIKey |
PCSKKIUURRTAEM-UHFFFAOYSA-N
|
|
| Synonyms |
6338-41-6; 5-Hydroxymethyl-2-furancarboxylic acid; 5-(hydroxymethyl)furan-2-carboxylic acid; Sumiki's acid; 5-Hydroxymethyl-2-furoic acid; 5-Hydroxymethyl-furan-2-carboxylic acid; 5-(Hydroxymethyl)-2-furoic acid; 2-Furancarboxylic acid, 5-(hydroxymethyl)-; 5-hydroxymethylfuran-2-carboxylic acid; 5-Hydroxymethyl-2-furoate; 2-Furoic acid, 5-(hydroxymethyl)-; 5-Hydroxymethyl-2-furancarboxylicacid; 5-hydroxymethylfuroic acid; 5-hydroxymethylfuranoic acid; CHEBI:89118; JI63TD4992; MFCD03274472; NSC-40739; Sumikis' Acid; NSC 40739; HMFCA; 5-hydroxymethylfuroate; 5-hydroxymethylfuranoate; BAS 00404252; EC-000.1550; DSSTox_CID_13098; DSSTox_RID_79050; DSSTox_GSID_33098; Oprea1_060549; Oprea1_518608; SCHEMBL50822; 5-(hydroxymethyl)-2-Furoate; UNII-JI63TD4992; CHEMBL468037; DTXSID5033098; 5-Hydroxymethyl-2-furanoic acid; 5-Hydroxymethyl-2-furancarboxylate; 5-hydroxymethylfurancarboxylic acid; HMS1681G08; ZINC327584; ALBB-004523; BCP25291; NSC40739; 5-Hydroxymethyl-furan-2-carboxylate; Tox21_201082; BBL015780; GEO-03105; s6081; STK256640; AKOS000505137; 5-(hydroxymethyl)- 2-Furancarboxylate; CS-W005241; HY-W005241; SB38045; 5-hydroxymethyl-2-furan-carboxylic acid; NCGC00091546-01; NCGC00091546-02; NCGC00091546-03; NCGC00260669-01; 65-I; AC-22968; AS-18462; SY062854; CAS-6338-41-6; DB-054467; AM20100567; BB 0256593; C2279; FT-0636101; H1750; EN300-91706; C20448; 338H416; A834352; 5-hydroxymethyl-2-furancarboxylic acid, AldrichCPR; J-517613; Q27161297; Z385458092
|
|
| CAS | 6338-41-6 | |
| PubChem CID | 80642 | |
| ChEMBL ID | CHEMBL468037 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 142.11 | ALogp: | 0.0 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 70.7 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.642 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.736 | MDCK Permeability: | 0.00005270 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.04 |
| Human Intestinal Absorption (HIA): | 0.041 | 20% Bioavailability (F20%): | 0.178 |
| 30% Bioavailability (F30%): | 0.989 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.173 | Plasma Protein Binding (PPB): | 54.07% |
| Volume Distribution (VD): | 0.255 | Fu: | 66.14% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.04 | CYP1A2-substrate: | 0.078 |
| CYP2C19-inhibitor: | 0.029 | CYP2C19-substrate: | 0.05 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.152 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.193 |
| CYP3A4-inhibitor: | 0.014 | CYP3A4-substrate: | 0.07 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.535 | Half-life (T1/2): | 0.941 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.044 | Human Hepatotoxicity (H-HT): | 0.106 |
| Drug-inuced Liver Injury (DILI): | 0.786 | AMES Toxicity: | 0.064 |
| Rat Oral Acute Toxicity: | 0.186 | Maximum Recommended Daily Dose: | 0.004 |
| Skin Sensitization: | 0.126 | Carcinogencity: | 0.794 |
| Eye Corrosion: | 0.007 | Eye Irritation: | 0.983 |
| Respiratory Toxicity: | 0.143 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
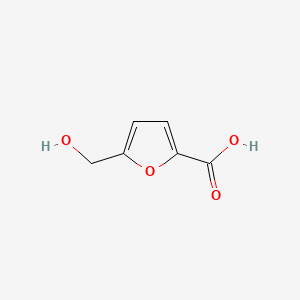
| ENC003372 | 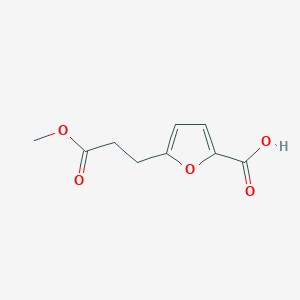 |
0.512 | D07HBX |  |
0.317 | ||
| ENC001019 |  |
0.486 | D0C4YC | 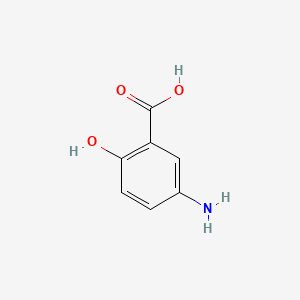 |
0.302 | ||
| ENC002334 | 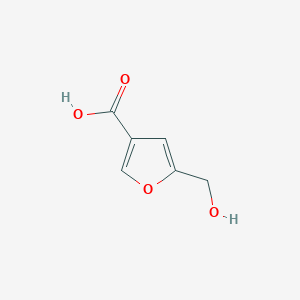 |
0.459 | D01WJL | 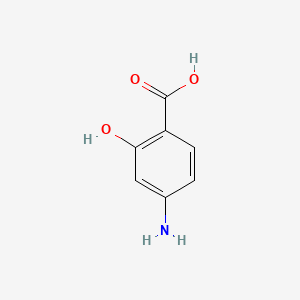 |
0.302 | ||
| ENC001324 | 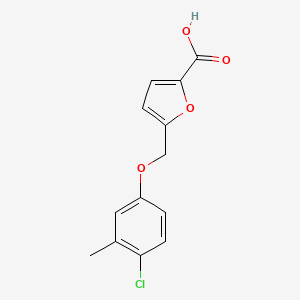 |
0.400 | D01CRB | 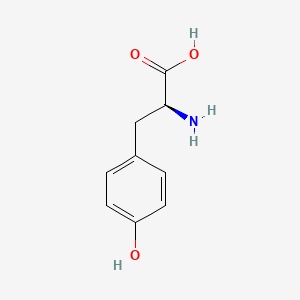 |
0.265 | ||
| ENC000162 | 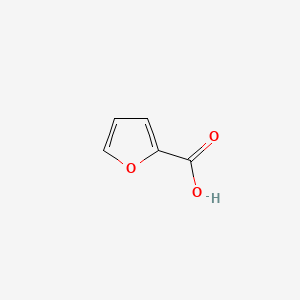 |
0.361 | D0N3UL | 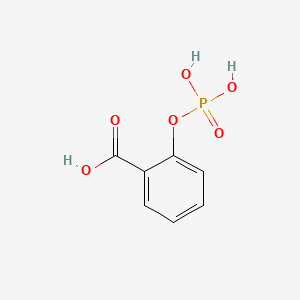 |
0.255 | ||
| ENC005253 | 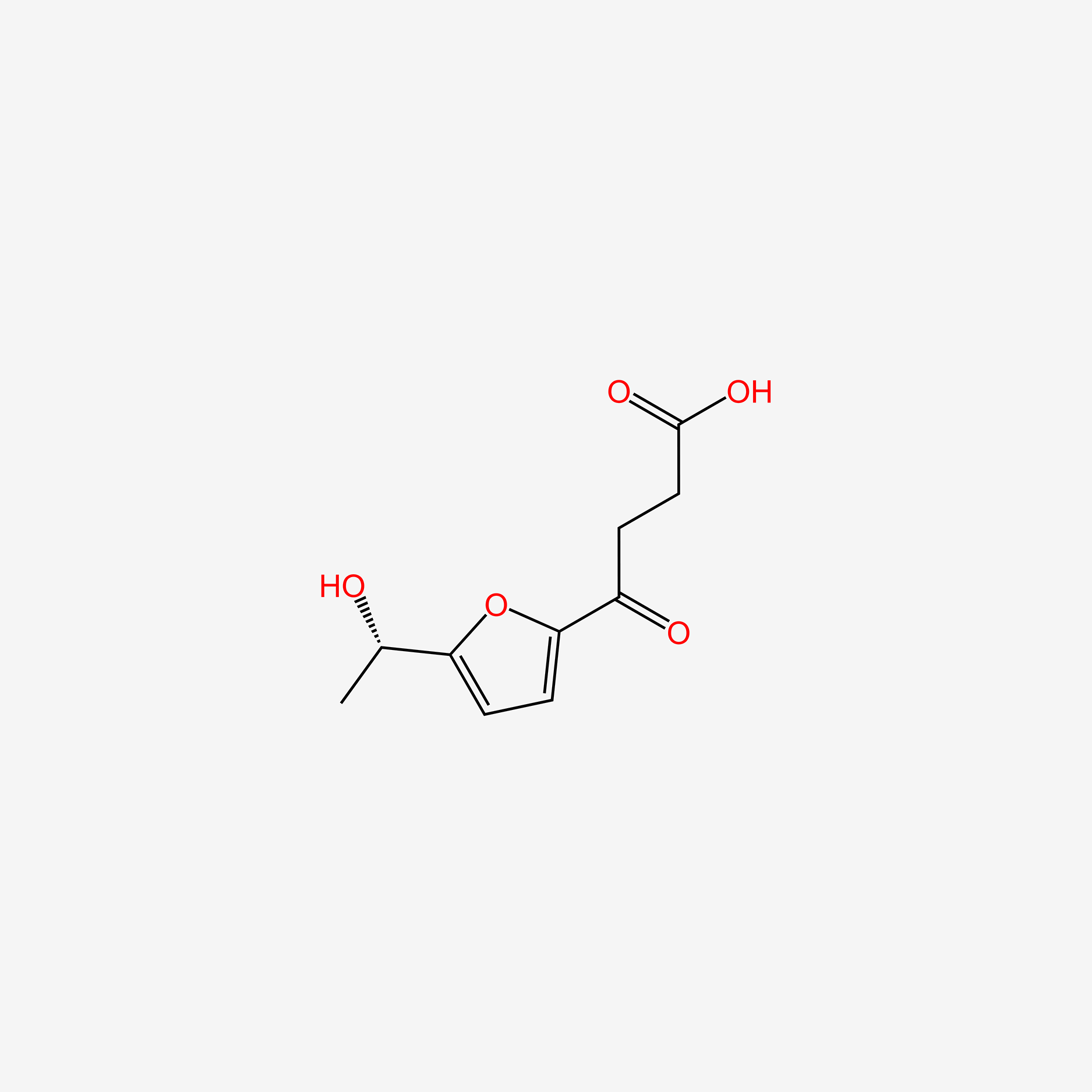 |
0.340 | D0B3QM | 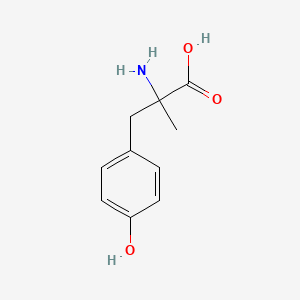 |
0.255 | ||
| ENC005755 | 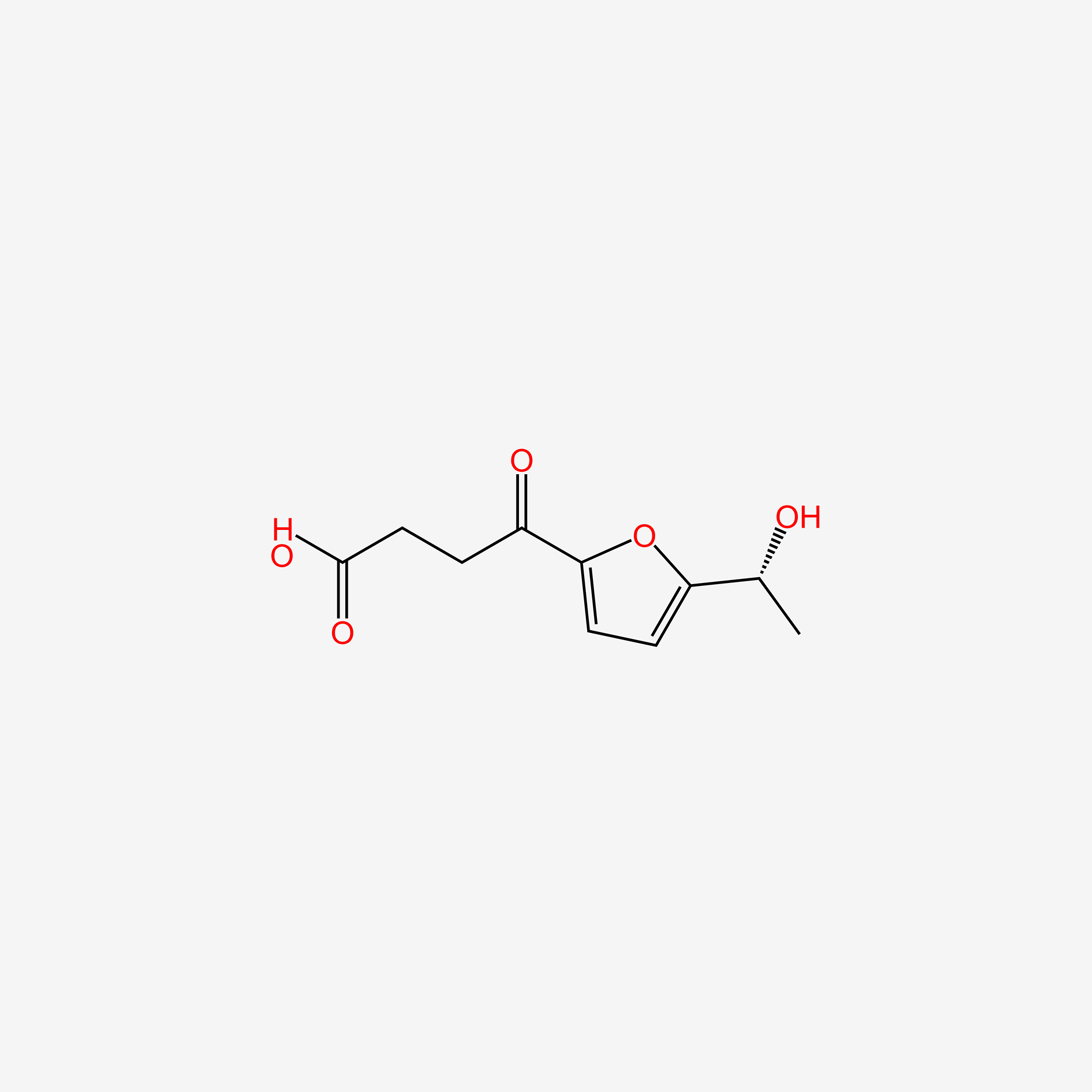 |
0.340 | D08HVR | 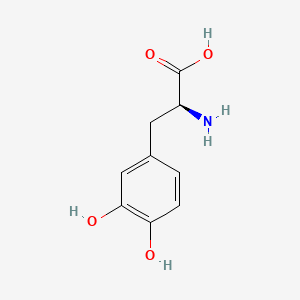 |
0.255 | ||
| ENC002433 | 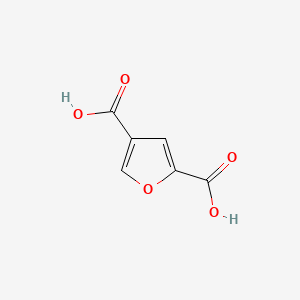 |
0.333 | D0BA6T | 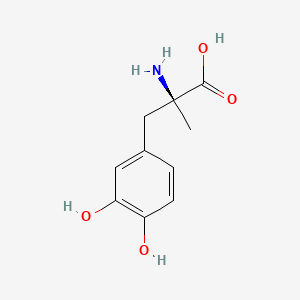 |
0.245 | ||
| ENC004365 | 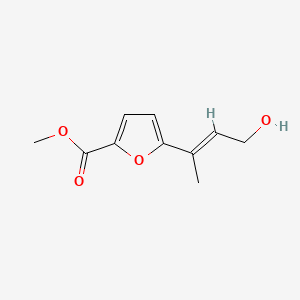 |
0.327 | D0V9EN |  |
0.240 | ||
| ENC003873 | 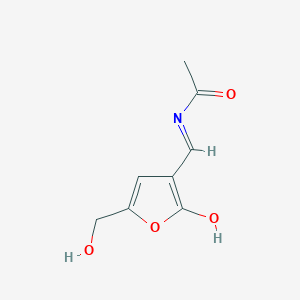 |
0.319 | D0GY5Z | 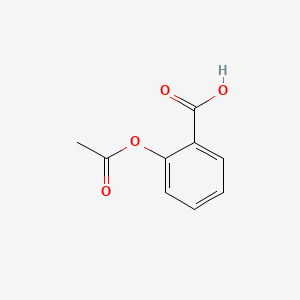 |
0.240 | ||