NPs Basic Information

|
Name |
Furfuryl alcohol
|
| Molecular Formula | C5H6O2 | |
| IUPAC Name* |
furan-2-ylmethanol
|
|
| SMILES |
C1=COC(=C1)CO
|
|
| InChI |
InChI=1S/C5H6O2/c6-4-5-2-1-3-7-5/h1-3,6H,4H2
|
|
| InChIKey |
XPFVYQJUAUNWIW-UHFFFAOYSA-N
|
|
| Synonyms |
FURFURYL ALCOHOL; 98-00-0; 2-Furanmethanol; furan-2-ylmethanol; 2-Furylmethanol; 2-Furancarbinol; 2-Furylcarbinol; Furfural alcohol; 2-Furanylmethanol; Furfuranol; 2-Furfuryl alcohol; Furfuralcohol; Furfurylalcohol; 2-(Hydroxymethyl)furan; 5-Hydroxymethylfuran; Furyl alcohol; 2-Hydroxymethylfuran; alpha-Furylcarbinol; Furylcarbinol; Furfurol; Furan-2-yl-methanol; 2-Furfurylalkohol; Furfurylcarb; Methanol, (2-furyl)-; (2-furyl)methanol; 2-hydroxymethylfurane; Furylcarbinol (VAN); Furan-2-methanol; 2-Furane-methanol; NCI-C56224; (furan-2-yl)methanol; FEMA No. 2491; 25212-86-6; 2-furanemethanol; NSC 8843; Qo furfuryl alcohol; .alpha.-Furylcarbinol; CHEBI:207496; .alpha.-Furfuryl alcohol; DTXSID2025347; D582054MUH; NSC-8843; Furfuryl alcohol, 98%; DSSTox_CID_5347; (2-FURYL)-METHANOL (FURFURYLALCOHOL); DSSTox_RID_77760; DSSTox_GSID_25347; Furanmethanol; 2-Furfurylalkohol [Czech]; CAS-98-00-0; CCRIS 2922; HSDB 711; EINECS 202-626-1; UN2874; BRN 0106291; furylmethanol; UNII-D582054MUH; AI3-01171; 2-furan carbinol; 2-Furfurylalcohol; FU2; alpha -Furylcarbinol; MFCD00003252; PFFA; (2-furyl)-Methanol; alpha-Furfuryl alcohol; Furfuryl alcohol [UN2874] [Poison]; 2-Hydroxymethyl-Furan; alpha -Furfuryl alcohol; Furfuryl alcohol, 8CI; 2- FURANCARBINOL; FURFURYLALCOHOLRESIN; 2- FURANYLMETHANOL; 2-Furfurylalkohol(CZECH); Epitope ID:136037; EC 202-626-1; furfuryl alcohol (furfurol); WLN: T5OJ B1Q; 5-17-03-00338 (Beilstein Handbook Reference); 2-Furane-methanol (furfurol); FURFURYL ALCOHOL [MI]; FURFURYL ALCOHOL [FCC]; CHEMBL308187; FURFURYL ALCOHOL [FHFI]; FURFURYL ALCOHOL [HSDB]; FURFURYL ALCOHOL [IARC]; CHEBI:53371; FEMA 2491; Furfuryl alcohol, >=97%, FG; NSC8843; 2-Furanmethanol (furfuryl alcohol); 2-Furylmethanol (ACD/Name 4.0); STR01021; ZINC1648266; Tox21_202102; Tox21_303093; Furfuryl alcohol, analytical standard; AKOS000119178; AM81811; UN 2874; Furfuryl alcohol [UN2874] [Poison]; Furfuryl alcohol, natural, >=95%, FG; NCGC00249166-01; NCGC00256987-01; NCGC00259651-01; 93793-62-5; DB-016149; F0076; FT-0626576; FT-0668910; EN300-19106; C20441; Q27335; A845784; J-521401; F0001-2310; Z104472794
|
|
| CAS | 98-00-0 | |
| PubChem CID | 7361 | |
| ChEMBL ID | CHEMBL308187 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 98.1 | ALogp: | 0.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 33.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 7 | QED Weighted: | 0.572 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.367 | MDCK Permeability: | 0.00010791 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.07 |
| 30% Bioavailability (F30%): | 0.01 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.931 | Plasma Protein Binding (PPB): | 66.11% |
| Volume Distribution (VD): | 3.282 | Fu: | 61.91% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.528 | CYP1A2-substrate: | 0.24 |
| CYP2C19-inhibitor: | 0.089 | CYP2C19-substrate: | 0.27 |
| CYP2C9-inhibitor: | 0.012 | CYP2C9-substrate: | 0.11 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.485 |
| CYP3A4-inhibitor: | 0.007 | CYP3A4-substrate: | 0.271 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.128 | Half-life (T1/2): | 0.898 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.047 | Human Hepatotoxicity (H-HT): | 0.122 |
| Drug-inuced Liver Injury (DILI): | 0.23 | AMES Toxicity: | 0.11 |
| Rat Oral Acute Toxicity: | 0.824 | Maximum Recommended Daily Dose: | 0.014 |
| Skin Sensitization: | 0.379 | Carcinogencity: | 0.851 |
| Eye Corrosion: | 0.228 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.776 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000546 | 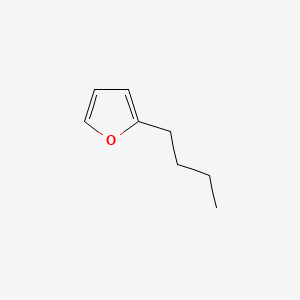 |
0.484 | D05OIS |  |
0.303 | ||
| ENC001133 | 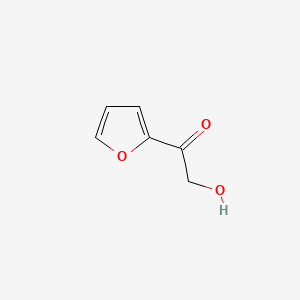 |
0.452 | D03OIW | 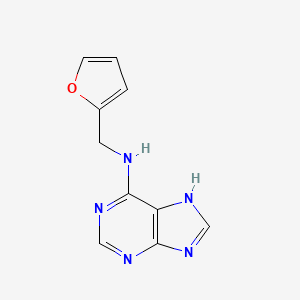 |
0.283 | ||
| ENC000533 | 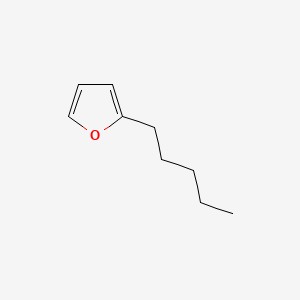 |
0.441 | D0PQ3G | 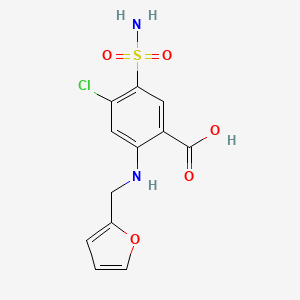 |
0.262 | ||
| ENC000162 | 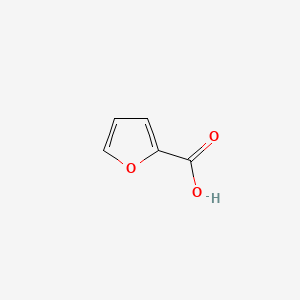 |
0.400 | D06NVJ | 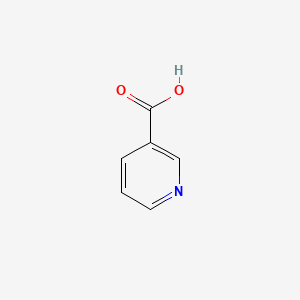 |
0.184 | ||
| ENC000190 | 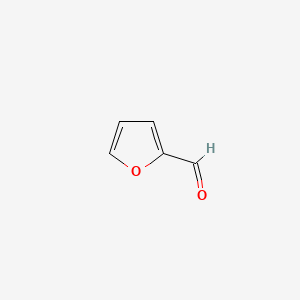 |
0.379 | D0S5LH | 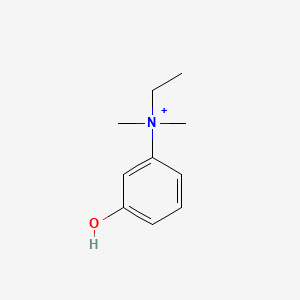 |
0.182 | ||
| ENC000534 | 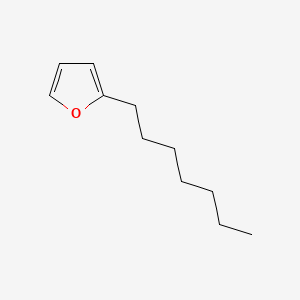 |
0.375 | D0R1CR |  |
0.178 | ||
| ENC000480 | 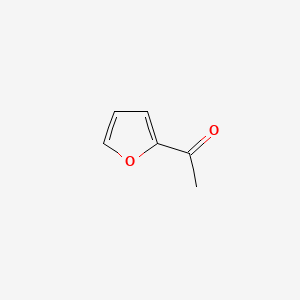 |
0.355 | D0O6IU | 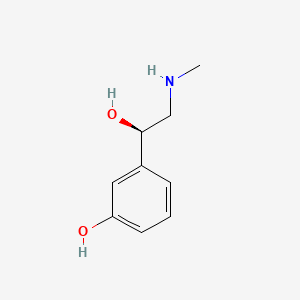 |
0.178 | ||
| ENC000678 | 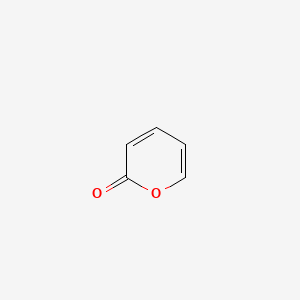 |
0.333 | D07HBX |  |
0.175 | ||
| ENC001019 | 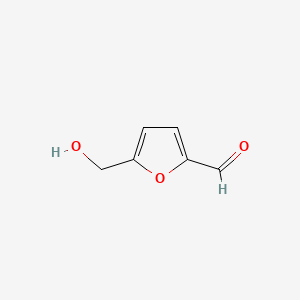 |
0.324 | D06BQU | 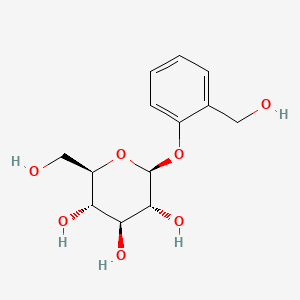 |
0.172 | ||
| ENC000748 | 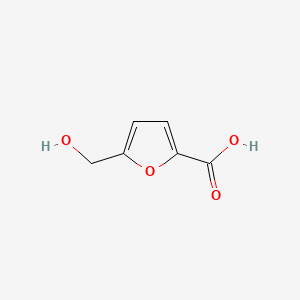 |
0.306 | D05BMG |  |
0.171 | ||