NPs Basic Information
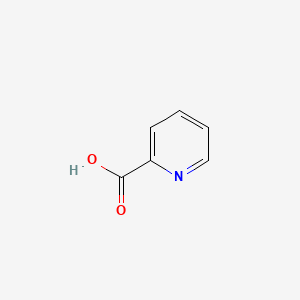
|
Name |
Picolinic acid
|
| Molecular Formula | C6H5NO2 | |
| IUPAC Name* |
pyridine-2-carboxylic acid
|
|
| SMILES |
C1=CC=NC(=C1)C(=O)O
|
|
| InChI |
InChI=1S/C6H5NO2/c8-6(9)5-3-1-2-4-7-5/h1-4H,(H,8,9)
|
|
| InChIKey |
SIOXPEMLGUPBBT-UHFFFAOYSA-N
|
|
| Synonyms |
picolinic acid; 98-98-6; 2-Picolinic acid; Pyridine-2-carboxylic acid; 2-Pyridinecarboxylic acid; 2-Carboxypyridine; o-Pyridinecarboxylic acid; alpha-Picolinic acid; alpha-Pyridinecarboxylic acid; PYRIDINECARBOXYLIC ACID; Acide picolique; Pyridine-carboxylique-2; 2-pyridine carboxylic acid; MFCD00006293; NSC 171; AI3-19242; PCL 016; Pyridinecarboxylic acid-(2); QZV2W997JQ; CHEMBL72628; .alpha.-Pyridinecarboxylic acid; CHEBI:28747; NSC 21209; NSC-171; NCGC00165993-01; Nicogamol; Nikogamol; 2-pyridinecarboxylate; DSSTox_CID_11903; DSSTox_RID_78896; DSSTox_GSID_31903; Acide picolique [French]; CAS-98-98-6; SMR000814709; NSC21209; Pyridine-carboxylique-2 [French]; EINECS 202-719-7; 32075-31-3; UNII-QZV2W997JQ; BRN 0109595; picolinicacid; picolinic-acid; picolic acid; CCRIS 8307; 2-picolic acid; A-Picolinic acid; 6PC; pyridine carboxylic acid; pyridine-carboxylic acid; 2-pyridincarboxylic acid; pyridinium 2-carboxylate; a-Pyridinecarboxylic acid; 2-pyridine carboxic acid; Pyridine-A-carboxylic acid; 2-pyridine-carboxylic Acid; PICOLINIC ACID [MI]; Oprea1_485360; SCHEMBL36055; 5-22-02-00003 (Beilstein Handbook Reference); MLS001335931; MLS001335932; Picolinic acid (PCL 016); Phenyl-(2-pyridyl)acetonitrile; NSC171; DTXSID7031903; SIOXPEMLGUPBBT-UHFFFAOYSA-; ZINC39905; HMS2232K05; BCP26613; CS-D1196; HY-I0660; STR00111; Tox21_112282; Tox21_202953; BDBM50000407; s6249; STL164340; AKOS000119032; Tox21_112282_1; AB00685; AC-2105; AM81303; PS-4253; NCGC00165993-02; NCGC00260499-01; 2-Picolinic acid, ReagentPlus(R), 99%; 88161-53-9; BP-13036; BP-21456; NCI60_001382; SY001169; DB-014967; FT-0608336; P0421; EN300-18459; 2-Picolinic acid, Vetec(TM) reagent grade, 98%; Q416682; C9DF3EBC-98F0-4165-BE9A-92F8EE33CDBA; W-100058; Z57968195; F2191-0113
|
|
| CAS | 98-98-6 | |
| PubChem CID | 1018 | |
| ChEMBL ID | CHEMBL72628 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 123.11 | ALogp: | 0.8 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 50.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.61 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.938 | MDCK Permeability: | 0.00001960 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.017 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.716 | Plasma Protein Binding (PPB): | 20.35% |
| Volume Distribution (VD): | 0.342 | Fu: | 73.01% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.032 | CYP1A2-substrate: | 0.088 |
| CYP2C19-inhibitor: | 0.03 | CYP2C19-substrate: | 0.052 |
| CYP2C9-inhibitor: | 0.016 | CYP2C9-substrate: | 0.134 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.122 |
| CYP3A4-inhibitor: | 0.012 | CYP3A4-substrate: | 0.057 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.807 | Half-life (T1/2): | 0.726 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.048 | Human Hepatotoxicity (H-HT): | 0.362 |
| Drug-inuced Liver Injury (DILI): | 0.959 | AMES Toxicity: | 0.018 |
| Rat Oral Acute Toxicity: | 0.558 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.289 | Carcinogencity: | 0.029 |
| Eye Corrosion: | 0.076 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.843 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000485 | 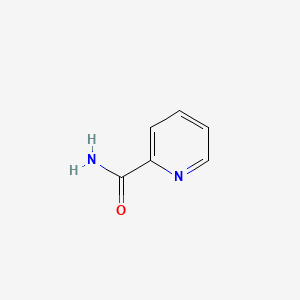 |
0.667 | D06NVJ | 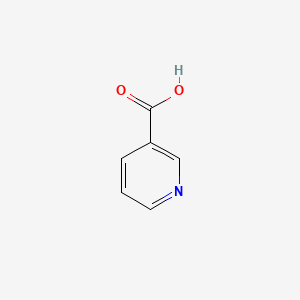 |
0.429 | ||
| ENC000585 | 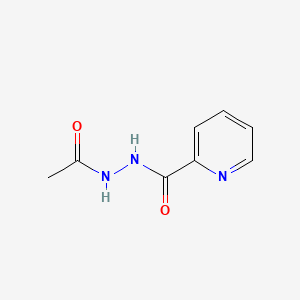 |
0.488 | D07HBX |  |
0.405 | ||
| ENC000013 | 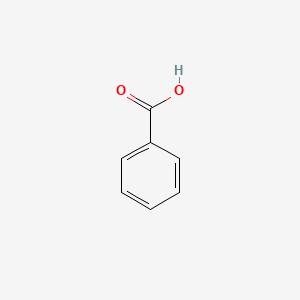 |
0.429 | D0F5ZM | 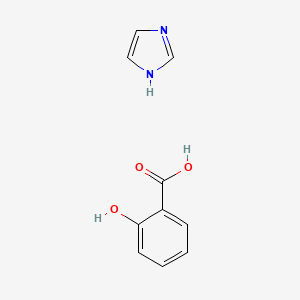 |
0.340 | ||
| ENC000348 | 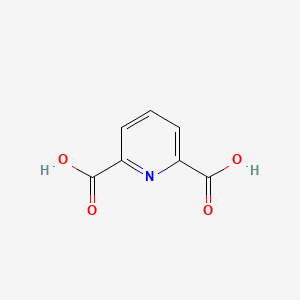 |
0.390 | D0GY5Z | 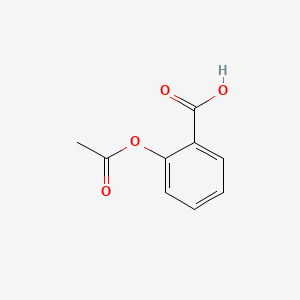 |
0.333 | ||
| ENC003520 | 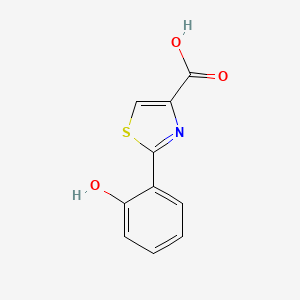 |
0.367 | D01ZJK |  |
0.333 | ||
| ENC000054 | 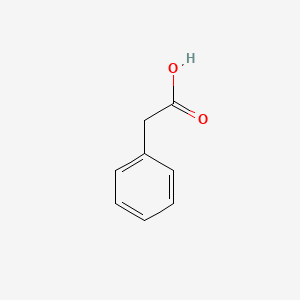 |
0.359 | D0N3UL | 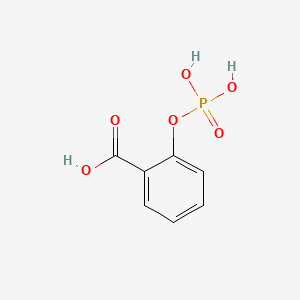 |
0.319 | ||
| ENC000055 | 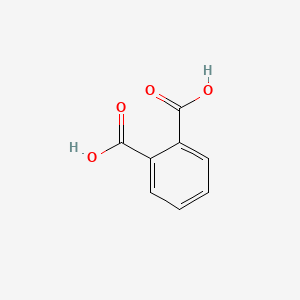 |
0.357 | D0R1CR |  |
0.318 | ||
| ENC000096 | 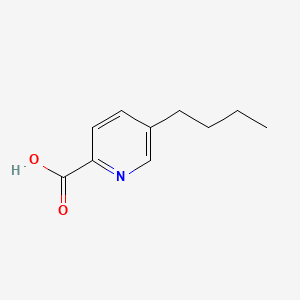 |
0.356 | D0S1NZ | 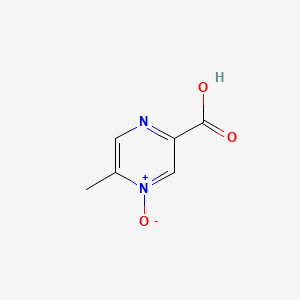 |
0.317 | ||
| ENC000162 | 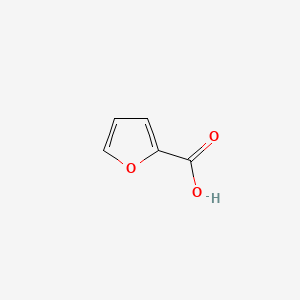 |
0.343 | D09SOA | 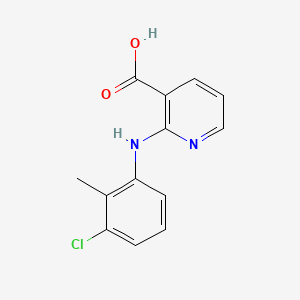 |
0.316 | ||
| ENC000439 |  |
0.343 | D0X9RY |  |
0.316 | ||