NPs Basic Information
|
Name |
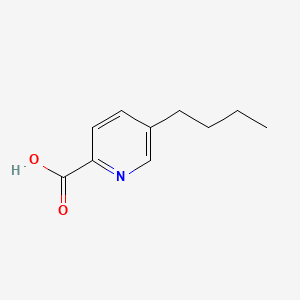
Fusaric acid
|
| Molecular Formula | C10H13NO2 | |
| IUPAC Name* |
5-butylpyridine-2-carboxylic acid
|
|
| SMILES |
CCCCC1=CN=C(C=C1)C(=O)O
|
|
| InChI |
InChI=1S/C10H13NO2/c1-2-3-4-8-5-6-9(10(12)13)11-7-8/h5-7H,2-4H2,1H3,(H,12,13)
|
|
| InChIKey |
DGMPVYSXXIOGJY-UHFFFAOYSA-N
|
|
| Synonyms |
fusaric acid; 5-Butylpicolinic acid; 536-69-6; 5-Butylpyridine-2-carboxylic acid; Fusarinic acid; 2-Pyridinecarboxylic acid, 5-butyl-; 5-Butyl-2-pyridinecarboxylic acid; Picolinic acid, 5-butyl-; 5-n-Butylpyridine-2-carboxylic acid; 5-Butyl-pyridine-2-carboxylic acid; CHEMBL24510; CHEBI:5199; JWJ963070N; NSC19870; TNP00268; NSC-19870; NCGC00015441-04; CAS-536-69-6; 5-Butylpyridine-3-carboxylic acid; HSDB 3487; EINECS 208-643-0; MFCD00006298; NSC 19870; BRN 0125804; UNII-JWJ963070N; Prestwick_233; 5-n-butylpicolinic acid; Prestwick0_000442; Prestwick1_000442; Prestwick2_000442; Prestwick3_000442; 5-n-butyl picolinic acid; Lopac-F-6513; DSSTox_CID_3085; FUSARIC ACID [MI]; FUSARIC ACID [JAN]; DSSTox_RID_76868; FUSARIC ACID [HSDB]; DSSTox_GSID_23085; Lopac0_000526; Oprea1_115508; WLN: T6NJ BVQ E4; 5-n-Butyl-2-picolinic acid; BSPBio_000484; 5-22-02-00384 (Beilstein Handbook Reference); MLS002153813; FUSARIC ACID [MART.]; SCHEMBL178006; SPBio_002423; BPBio1_000534; DTXSID5023085; HMS1569I06; HMS2096I06; HMS2230M05; HMS3261J13; HMS3369P03; ZINC1531682; Tox21_110149; Tox21_500526; BDBM50000439; STL564384; AKOS015891748; CCG-204616; Fusaric acid, from Gibberella fujikuroi; LP00526; SDCCGSBI-0050509.P002; NCGC00015441-01; NCGC00015441-02; NCGC00015441-03; NCGC00015441-05; NCGC00015441-06; NCGC00015441-07; NCGC00015441-08; NCGC00015441-10; NCGC00015441-15; NCGC00093919-01; NCGC00093919-02; NCGC00093919-03; NCGC00261211-01; AS-57621; SMR001233184; DB-052375; HY-128483; CS-0099145; EU-0100526; F0227; FT-0626585; A19903; F 6513; F-9000; T72585; 5-Butyl-pyridine-2-carboxylic acid (fusaric acid); Q905703; SR-01000075634; SR-01000075634-1; BRD-K87049188-001-03-6; Fusaric acid, for HPLC derivatization, >=99.0% (HPLC); 4-(ACETYLAMINO)-6-NITRO-1,3-BENZENEDICARBOXYLICACID; CQV
|
|
| CAS | 536-69-6 | |
| PubChem CID | 3442 | |
| ChEMBL ID | CHEMBL24510 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 179.22 | ALogp: | 2.6 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 50.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.773 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.638 | MDCK Permeability: | 0.00002200 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.333 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.293 | Plasma Protein Binding (PPB): | 79.25% |
| Volume Distribution (VD): | 0.35 | Fu: | 19.73% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.162 | CYP1A2-substrate: | 0.136 |
| CYP2C19-inhibitor: | 0.079 | CYP2C19-substrate: | 0.06 |
| CYP2C9-inhibitor: | 0.088 | CYP2C9-substrate: | 0.315 |
| CYP2D6-inhibitor: | 0.021 | CYP2D6-substrate: | 0.124 |
| CYP3A4-inhibitor: | 0.02 | CYP3A4-substrate: | 0.06 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.581 | Half-life (T1/2): | 0.702 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.126 | Human Hepatotoxicity (H-HT): | 0.427 |
| Drug-inuced Liver Injury (DILI): | 0.965 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.925 | Maximum Recommended Daily Dose: | 0.027 |
| Skin Sensitization: | 0.156 | Carcinogencity: | 0.055 |
| Eye Corrosion: | 0.013 | Eye Irritation: | 0.966 |
| Respiratory Toxicity: | 0.839 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002111 | 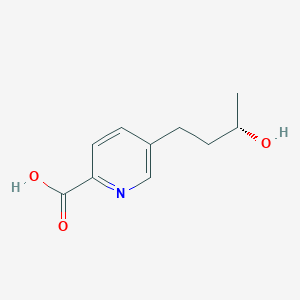 |
0.644 | D0S1NZ | 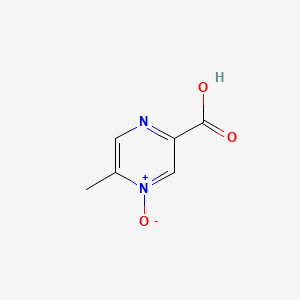 |
0.354 | ||
| ENC004033 | 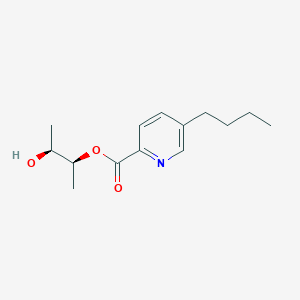 |
0.604 | D02HXS | 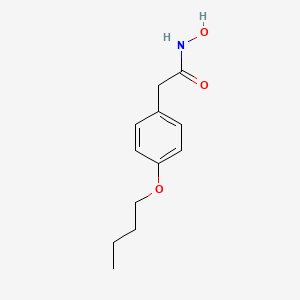 |
0.350 | ||
| ENC004034 | 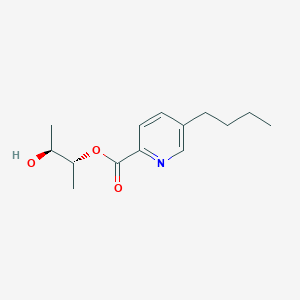 |
0.604 | D0T7US | 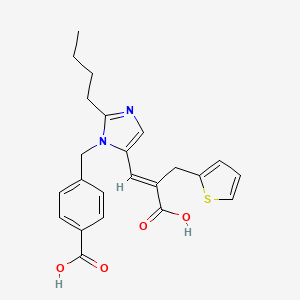 |
0.301 | ||
| ENC004035 | 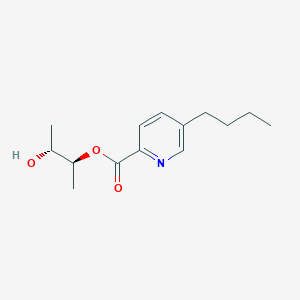 |
0.604 | D0P2GK | 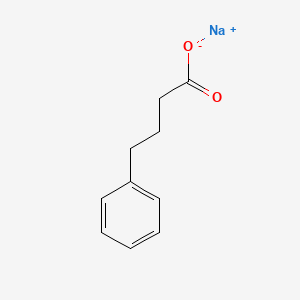 |
0.291 | ||
| ENC004036 | 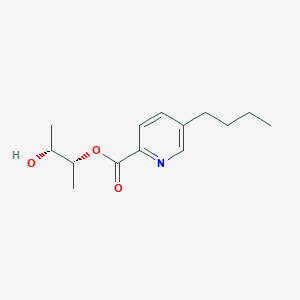 |
0.604 | D0V8QT | 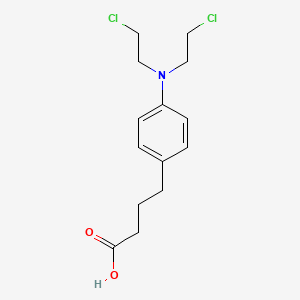 |
0.290 | ||
| ENC004143 | 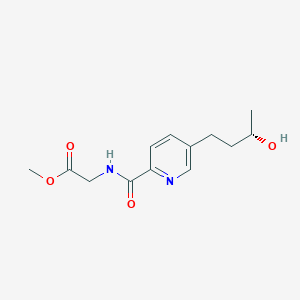 |
0.419 | D0B3QM | 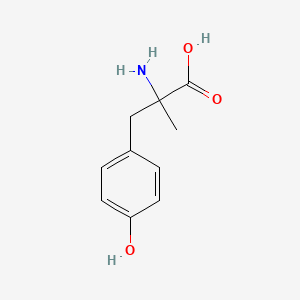 |
0.281 | ||
| ENC000301 | 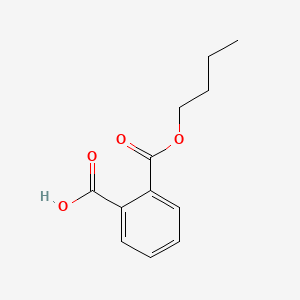 |
0.356 | D0L7FM | 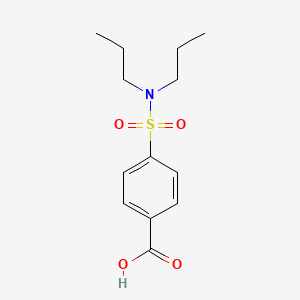 |
0.279 | ||
| ENC000056 | 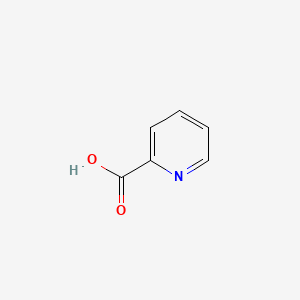 |
0.356 | D0L7UQ | 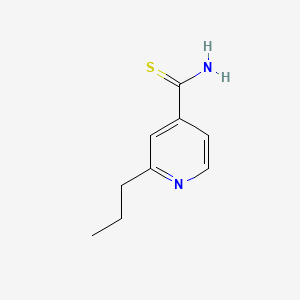 |
0.278 | ||
| ENC002450 | 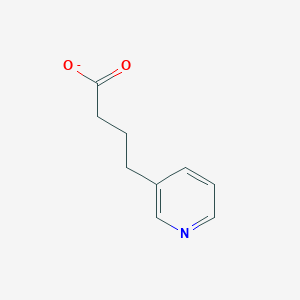 |
0.346 | D02AQY | 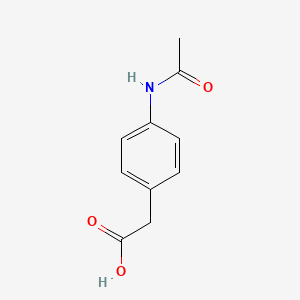 |
0.276 | ||
| ENC002237 | 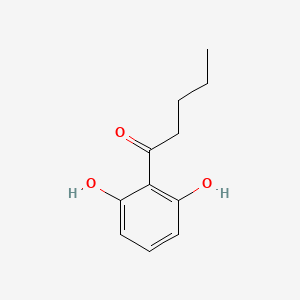 |
0.345 | D0BA6T | 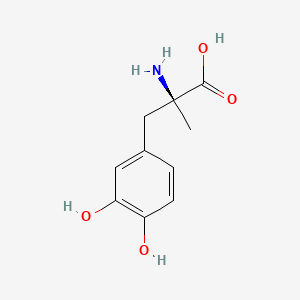 |
0.271 | ||