NPs Basic Information
|
Name |
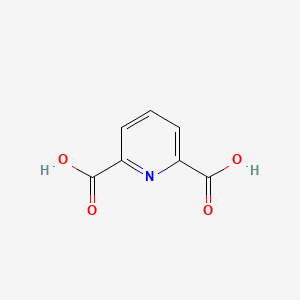
2,6-Pyridinedicarboxylic acid
|
| Molecular Formula | C7H5NO4 | |
| IUPAC Name* |
pyridine-2,6-dicarboxylic acid
|
|
| SMILES |
C1=CC(=NC(=C1)C(=O)O)C(=O)O
|
|
| InChI |
InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-5(8-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
|
|
| InChIKey |
WJJMNDUMQPNECX-UHFFFAOYSA-N
|
|
| Synonyms |
2,6-Pyridinedicarboxylic acid; 499-83-2; PYRIDINE-2,6-DICARBOXYLIC ACID; Dipicolinic acid; 2,6-Dipicolinic acid; Dipicolinate; 2,6-Dicarboxypyridine; MFCD00006299; 2,6-pyridinedicarboxylate; UE81S5CQ0G; CHEMBL284104; CHEBI:46837; NSC-176; 2,6-Pyridinedicarboxylic acid, 99%; NSC 176; EINECS 207-894-3; UNII-UE81S5CQ0G; 2,6-pyridine dicarboxylic acid; pyridine-2; pydcH2; 4ih3; pyridine carboxylate, 6d; DSSTox_CID_2043; DSSTox_RID_76466; DSSTox_GSID_22043; Oprea1_533632; SCHEMBL34595; 2,6-DIPICLINIC ACID; MLS000080748; pyridine-2,6-dicarboxlic acid; 6-CARBOXYPICOLINIC ACID; IFLab1_001781; NSC176; Dipicolinic acid, Beauveria sp.; DTXSID7022043; BDBM26116; 2,6-DI-CARBOXY-PYRIDINE; Pyridinedicarboxylic acid-(2,6); HMS1417A21; HMS2231H20; ZINC105246; ACT07463; Tox21_301129; AC-704; BBL012080; CCG-44216; CL0252; STK092939; PYRIDINE-2,6-DICARBOXYLICACID; 2,6-DICARBOXYPYRIDINE [INCI]; AKOS000112829; AM82010; DB04267; PS-8736; NCGC00071864-02; NCGC00255028-01; CAS-499-83-2; SMR000034075; SY001460; DB-015930; A7431; CS-0016012; EU-0033484; FT-0610741; P0554; EN300-18133; Q417164; 2,6-Pyridinedicarboxylic acid-2,6-dipicolinic acid; SR-01000600024-2; W-105996; L-042,134; Z57202012; B63A70CE-B9AB-4EA2-834A-6C7634226BB0; F0451-0137; 2,6-Pyridinedicarboxylic acid, for ion chromatography, >=99.5% (T)
|
|
| CAS | 499-83-2 | |
| PubChem CID | 10367 | |
| ChEMBL ID | CHEMBL284104 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 167.12 | ALogp: | 0.6 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 87.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.682 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.674 | MDCK Permeability: | 0.00000559 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.011 |
| 30% Bioavailability (F30%): | 0.394 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.217 | Plasma Protein Binding (PPB): | 41.07% |
| Volume Distribution (VD): | 0.298 | Fu: | 60.22% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.036 | CYP1A2-substrate: | 0.051 |
| CYP2C19-inhibitor: | 0.023 | CYP2C19-substrate: | 0.033 |
| CYP2C9-inhibitor: | 0.007 | CYP2C9-substrate: | 0.055 |
| CYP2D6-inhibitor: | 0.028 | CYP2D6-substrate: | 0.051 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.013 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.326 | Half-life (T1/2): | 0.81 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.077 | Human Hepatotoxicity (H-HT): | 0.422 |
| Drug-inuced Liver Injury (DILI): | 0.922 | AMES Toxicity: | 0.017 |
| Rat Oral Acute Toxicity: | 0.25 | Maximum Recommended Daily Dose: | 0.004 |
| Skin Sensitization: | 0.163 | Carcinogencity: | 0.012 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.929 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000055 | 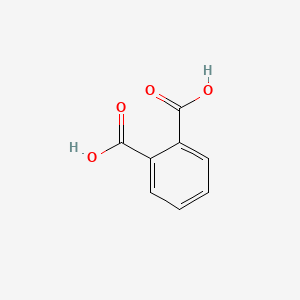 |
0.455 | D07HBX |  |
0.341 | ||
| ENC000202 | 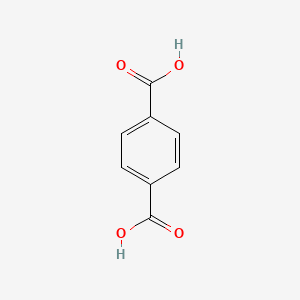 |
0.422 | D0GY5Z | 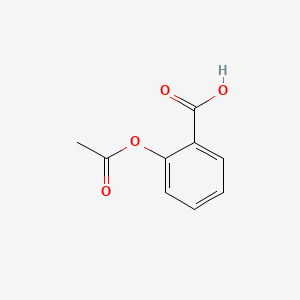 |
0.340 | ||
| ENC000056 | 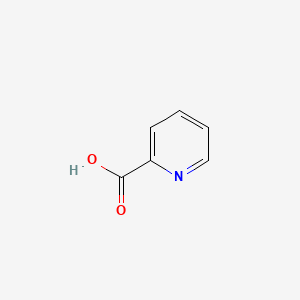 |
0.390 | D0N3UL | 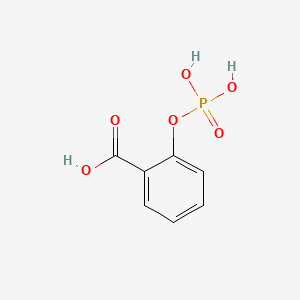 |
0.302 | ||
| ENC000684 | 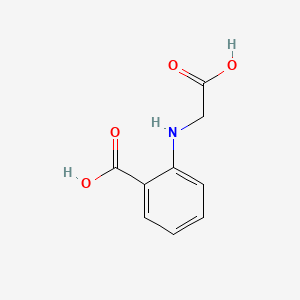 |
0.373 | D01WJL | 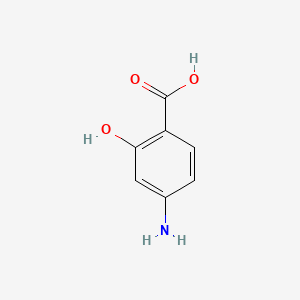 |
0.298 | ||
| ENC002433 | 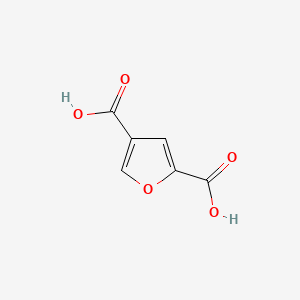 |
0.356 | D0C4YC | 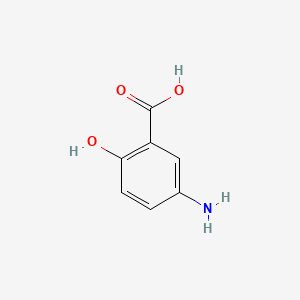 |
0.298 | ||
| ENC000390 | 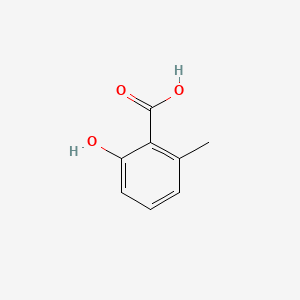 |
0.356 | D06NVJ | 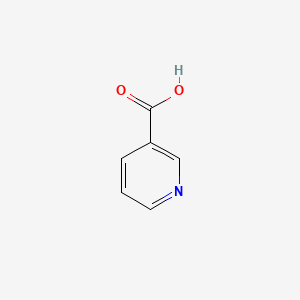 |
0.295 | ||
| ENC003520 |  |
0.345 | D06MRT |  |
0.290 | ||
| ENC000073 |  |
0.340 | D0F5ZM |  |
0.276 | ||
| ENC002111 | 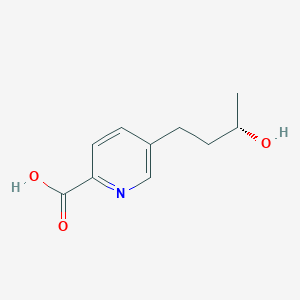 |
0.321 | D02AQY | 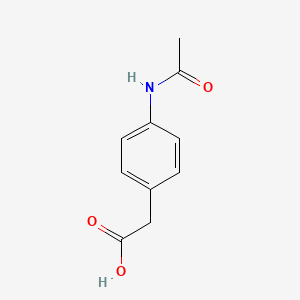 |
0.273 | ||
| ENC006051 | 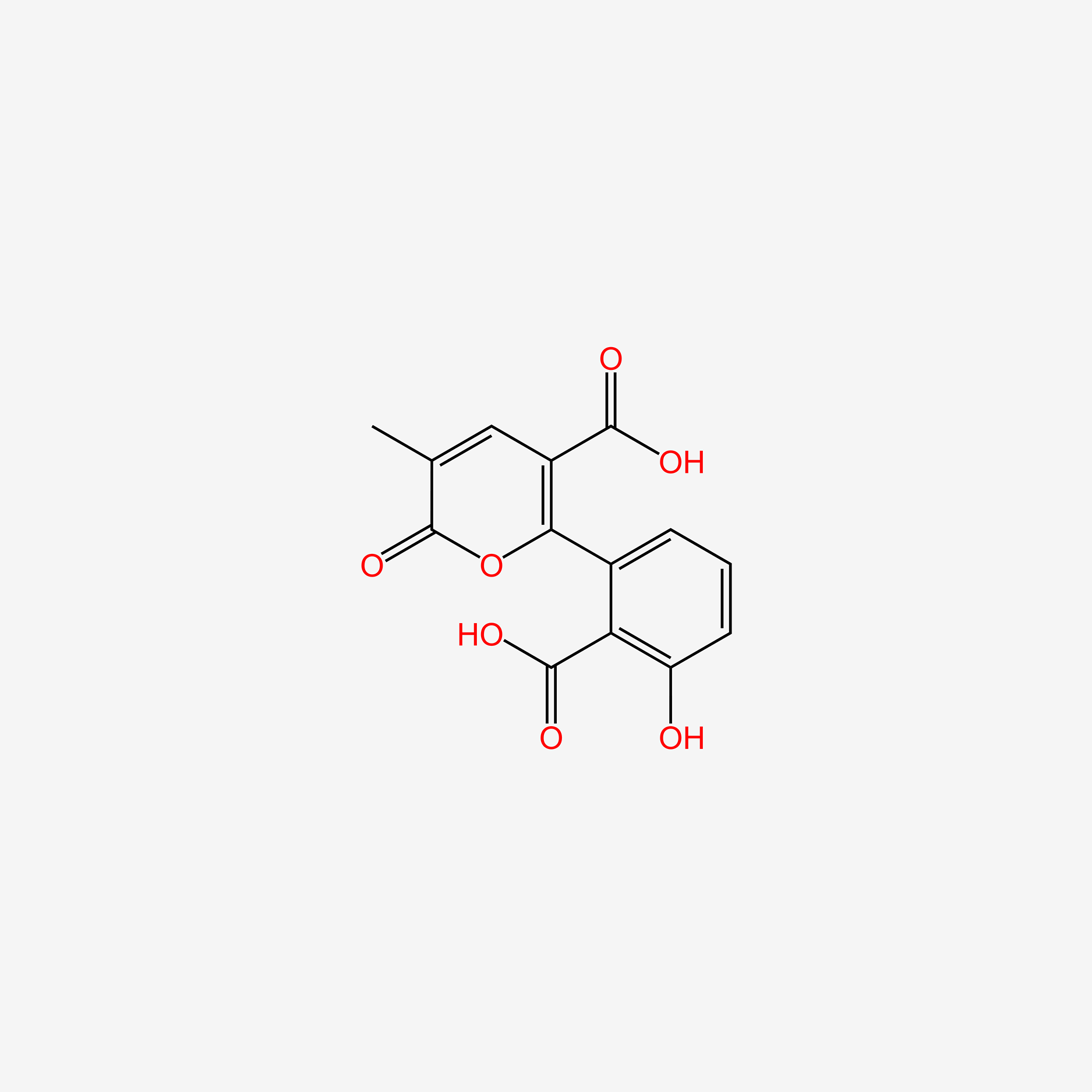 |
0.313 | D0S1NZ | 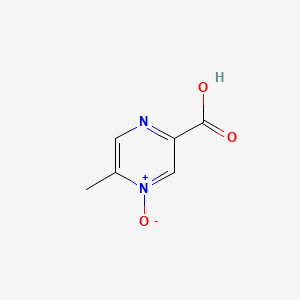 |
0.271 | ||