NPs Basic Information
|
Name |
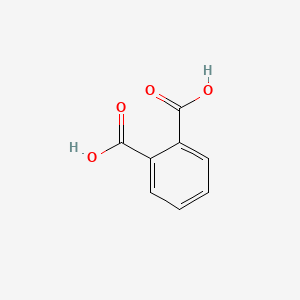
Phthalic acid
|
| Molecular Formula | C8H6O4 | |
| IUPAC Name* |
phthalic acid
|
|
| SMILES |
C1=CC=C(C(=C1)C(=O)O)C(=O)O
|
|
| InChI |
InChI=1S/C8H6O4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H,(H,9,10)(H,11,12)
|
|
| InChIKey |
XNGIFLGASWRNHJ-UHFFFAOYSA-N
|
|
| Synonyms |
phthalic acid; 88-99-3; 1,2-benzenedicarboxylic acid; o-phthalic acid; benzene-1,2-dicarboxylic acid; Pathalic acid; o-dicarboxybenzene; o-benzenedicarboxylic acid; Acide phtalique; PHTHALICACID; Kyselina ftalova; ortho-phthalic acid; Orthophthalic acid; MFCD00002467; Sunftal 20; CHEMBL1045; 6O7F7IX66E; CHEBI:29069; NSC-5348; Benzene-1,2-dicarboxylic Acid (Phthalic Acid); Acide phtalique [French]; Kyselina ftalova [Czech]; CCRIS 1446; HSDB 1339; NSC 5348; EINECS 201-873-2; BRN 0608199; UNII-6O7F7IX66E; Alizarinate; Naphthalinate; Phthalinate; Alizarinic acid; Phthalinic acid; Pathalc acd; AI3-02409; Naphthalinic acid; o-Carboxybenzoate; 4kww; phthalsäure; o-Carboxybenzoic acid; o-Benzenedicarboxylate; 1,2-benzenedioic acid; Phthalic acid, ~99%; WLN: QVR BVQ; Phthalate standard for IC; DSSTox_CID_1484; Phthalic acid, 99.5%; bmse000391; EC 201-873-2; PHTHALIC ACID [MI]; SCHEMBL1808; DSSTox_RID_76178; DSSTox_GSID_21484; PHTHALIC ACID [HSDB]; 4-09-00-03167 (Beilstein Handbook Reference); MLS002152931; PHTHALIC ACID [USP-RS]; DTXSID8021484; ZINC90750; NSC5348; HMS3039E17; HMS3604J03; Phthalic acid, analytical standard; BCP15370; HY-I0508; STR06656; Phthalic acid, reagent grade, 98%; Tox21_200915; BDBM50080272; PHTHALIC ACID [USP IMPURITY]; s6215; STL168879; AKOS000118898; DB02746; CAS-88-99-3; NCGC00090869-01; NCGC00090869-02; NCGC00258469-01; Phthalic acid, ACS reagent, >=99.5%; AC-14464; BP-21159; SMR001224528; CS-0009407; FLUORESCEIN IMPURITY B [EP IMPURITY]; FT-0622644; FT-0673874; P0287; Phthalic acid, SAJ first grade, >=99.0%; EN300-17992; Phthalic acid, SAJ special grade, >=99.0%; C01606; Phthalic acid, Vetec(TM) reagent grade, 98%; Phthalic acid, puriss. p.a., >=99.5% (T); AB-131/40237186; Q423876; FLUORESCEIN SODIUM IMPURITY B [EP IMPURITY]; J-523870; Z57127456; F3110-2832; Phthalic acid, European Pharmacopoeia (EP) Reference Standard; Phthalic acid, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 88-99-3 | |
| PubChem CID | 1017 | |
| ChEMBL ID | CHEMBL1045 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 166.13 | ALogp: | 0.7 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 12 | QED Weighted: | 0.698 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.74 | MDCK Permeability: | 0.00001020 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.071 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.897 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.399 | Plasma Protein Binding (PPB): | 53.42% |
| Volume Distribution (VD): | 0.204 | Fu: | 34.12% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.039 | CYP1A2-substrate: | 0.043 |
| CYP2C19-inhibitor: | 0.043 | CYP2C19-substrate: | 0.039 |
| CYP2C9-inhibitor: | 0.111 | CYP2C9-substrate: | 0.058 |
| CYP2D6-inhibitor: | 0.037 | CYP2D6-substrate: | 0.055 |
| CYP3A4-inhibitor: | 0.022 | CYP3A4-substrate: | 0.025 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.376 | Half-life (T1/2): | 0.91 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.108 | Human Hepatotoxicity (H-HT): | 0.361 |
| Drug-inuced Liver Injury (DILI): | 0.88 | AMES Toxicity: | 0.019 |
| Rat Oral Acute Toxicity: | 0.111 | Maximum Recommended Daily Dose: | 0.002 |
| Skin Sensitization: | 0.374 | Carcinogencity: | 0.01 |
| Eye Corrosion: | 0.02 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.219 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000684 | 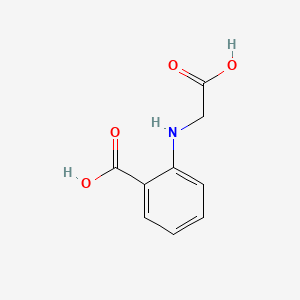 |
0.591 | D07HBX |  |
0.595 | ||
| ENC000073 | 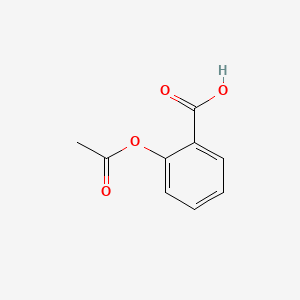 |
0.558 | D0GY5Z | 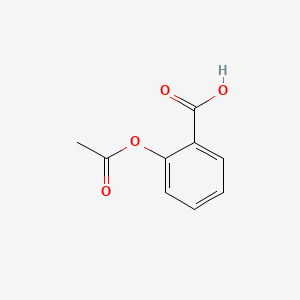 |
0.558 | ||
| ENC000301 | 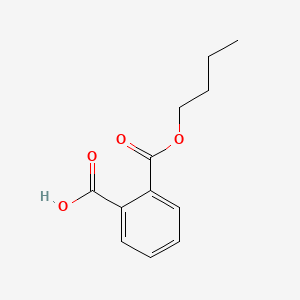 |
0.551 | D0N3UL | 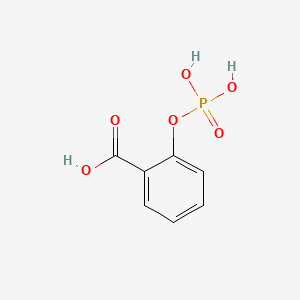 |
0.500 | ||
| ENC001356 | 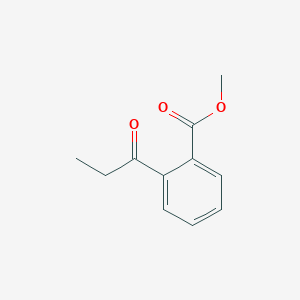 |
0.458 | D0Y0JH | 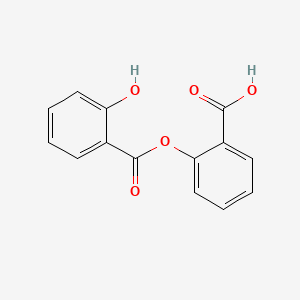 |
0.466 | ||
| ENC000299 | 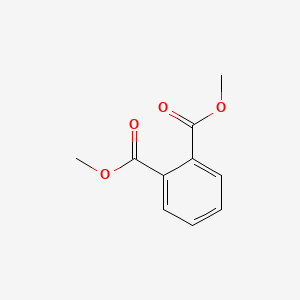 |
0.458 | D0F5ZM | 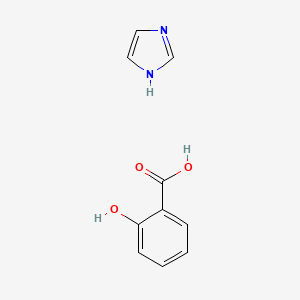 |
0.423 | ||
| ENC000348 | 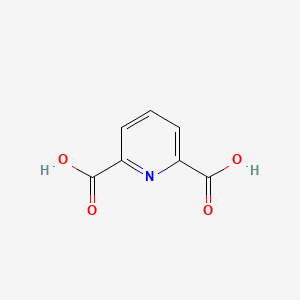 |
0.455 | D0G2MH | 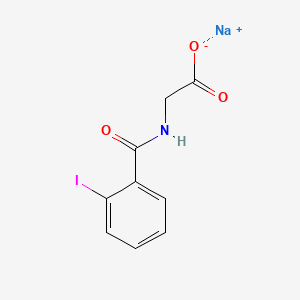 |
0.392 | ||
| ENC000202 | 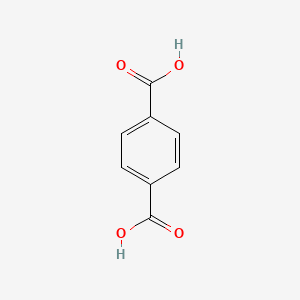 |
0.455 | D0C4YC | 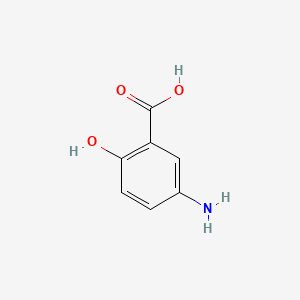 |
0.356 | ||
| ENC003916 | 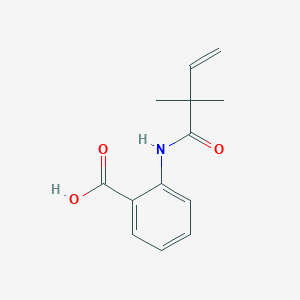 |
0.453 | D01WJL | 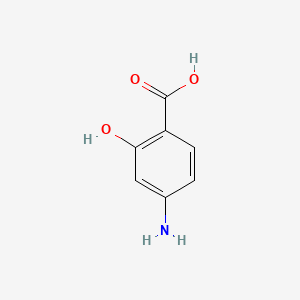 |
0.356 | ||
| ENC000544 | 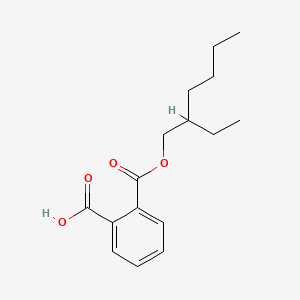 |
0.450 | D00KRE | 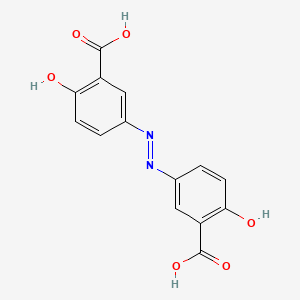 |
0.353 | ||
| ENC000108 | 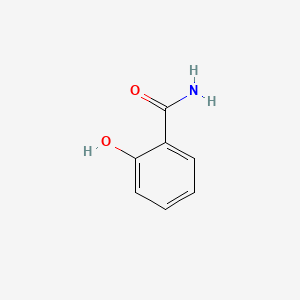 |
0.439 | D05FTJ | 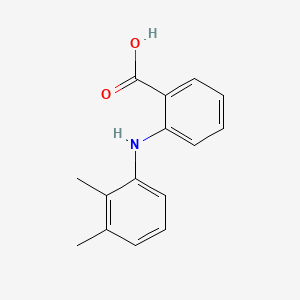 |
0.344 | ||