NPs Basic Information
|
Name |
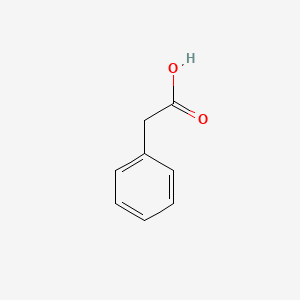
Phenylacetic Acid
|
| Molecular Formula | C8H8O2 | |
| IUPAC Name* |
2-phenylacetic acid
|
|
| SMILES |
C1=CC=C(C=C1)CC(=O)O
|
|
| InChI |
InChI=1S/C8H8O2/c9-8(10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,9,10)
|
|
| InChIKey |
WLJVXDMOQOGPHL-UHFFFAOYSA-N
|
|
| Synonyms |
PHENYLACETIC ACID; 2-Phenylacetic acid; Benzeneacetic acid; 103-82-2; Phenylethanoic acid; alpha-Toluic acid; Acetic acid, phenyl-; phenylacetate; Benzenacetic acid; Benzylformic acid; Phenyllacetic acid; Benzylcarboxylic acid; PHENYL ACETIC ACID; Kyselina fenyloctova; Phenylacetic acid (natural); .alpha.-Toluic acid; Kyselina fenyloctova [Czech]; omega-Phenylacetic acid; FEMA No. 2878; .omega.-Phenylacetic acid; NSC 125718; BRN 1099647; CHEBI:30745; AI3-08920; PHENYL-ACETIC ACID; CHEMBL1044; ER5I1W795A; Benzeneacetate; MFCD00004313; NSC125718; NSC-125718; NCGC00159477-02; 51146-16-8; DSSTox_CID_1656; DSSTox_RID_76268; DSSTox_GSID_21656; 1173020-54-6; 17303-65-0; CAS-103-82-2; HSDB 5010; EINECS 203-148-6; UNII-ER5I1W795A; Phenylacetic; Phenylethanoate; Phenylessigsaure; w-Phenylacetate; alpha-Toluate; phenylactic acid; a-Toluate; a-Toluic acid; Benzeneacetiic acid; omega-Phenylacetate; organic white solid; w-Phenylacetic acid; Phenylacetate, XIX; 2-phenyl-acetic acid; Phenylacetic acid, 99%; bmse000220; Epitope ID:116202; EC 203-148-6; SCHEMBL1459; 4-09-00-01614 (Beilstein Handbook Reference); Phenyl-[13C6]-acetic acid; PHENYLACETIC ACID [MI]; PHENYLACETIC ACID [FCC]; DTXSID2021656; PHENYLACETIC ACID [FHFI]; PHENYLACETIC ACID [HSDB]; BDBM16419; ZINC388462; Phenylacetic acid_GurudeebanSatyavani; Tox21_113042; Tox21_200533; NSC139637; Phenylacetic acid, natural, >=99%; STK297835; Phenylacetic acid, analytical standard; AKOS000291351; Tox21_113042_1; DB09269; DL-0063; NSC-139637; Phenylacetic acid, >=99%, FCC, FG; NCGC00159477-03; NCGC00159477-05; NCGC00258087-01; BP-11383; NCI60_000596; NCI60_002571; Phenylacetic acid, natural, >=99%, FG; ASTUGENAL COMPONENT PHENYLACETIC ACID; DB-003759; DB-055176; FT-0641197; FT-0701063; Phenylacetic acid, plant cell culture tested; TROPICAMIDE IMPURITY D [EP IMPURITY]; C07086; Q410842; ANTINEOPLASTON AS 2-1 COMPONENT PHENYLACETIC ACID; ANTINEOPLASTON AS2-1 COMPONENT PHENYLACETIC ACID; BENZYLPENICILLIN SODIUM IMPURITY B [EP IMPURITY]; BENZYLPENICILLIN POTASSIUM IMPURITY B [EP IMPURITY]; PROCAINE BENZYLPENICILLIN IMPURITY E [EP IMPURITY]; 8727557E-AA75-49E9-8E5A-7A2412D71888; Tropicamide impurity D (Phenylacetic acid - Drug Precursor), European Pharmacopoeia (EP) Reference Standard
|
|
| CAS | 103-82-2 | |
| PubChem CID | 999 | |
| ChEMBL ID | CHEMBL1044 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.15 | ALogp: | 1.4 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.673 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.793 | MDCK Permeability: | 0.00006250 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.051 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.364 | Plasma Protein Binding (PPB): | 68.38% |
| Volume Distribution (VD): | 0.177 | Fu: | 21.68% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.045 | CYP1A2-substrate: | 0.084 |
| CYP2C19-inhibitor: | 0.031 | CYP2C19-substrate: | 0.484 |
| CYP2C9-inhibitor: | 0.026 | CYP2C9-substrate: | 0.853 |
| CYP2D6-inhibitor: | 0.004 | CYP2D6-substrate: | 0.197 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.197 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.405 | Half-life (T1/2): | 0.877 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.016 | Human Hepatotoxicity (H-HT): | 0.128 |
| Drug-inuced Liver Injury (DILI): | 0.874 | AMES Toxicity: | 0.027 |
| Rat Oral Acute Toxicity: | 0.046 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.4 | Carcinogencity: | 0.112 |
| Eye Corrosion: | 0.958 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.036 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000218 | 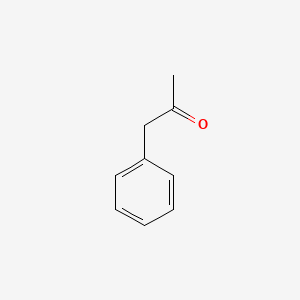 |
0.697 | D0R1CR |  |
0.605 | ||
| ENC000219 | 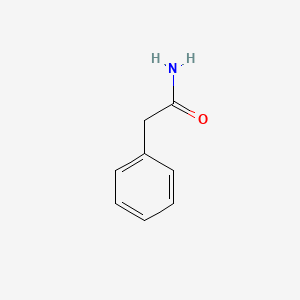 |
0.697 | D05OIS |  |
0.594 | ||
| ENC005854 |  |
0.697 | D07ONP | 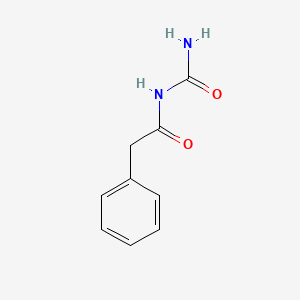 |
0.561 | ||
| ENC000004 | 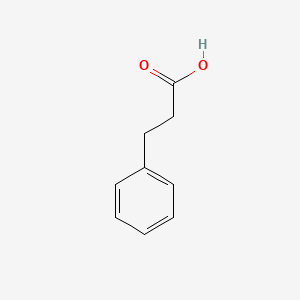 |
0.686 | D0Y7EM | 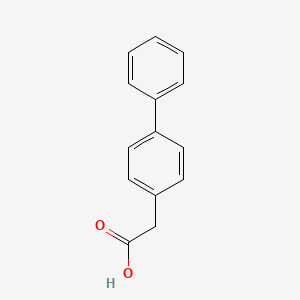 |
0.542 | ||
| ENC000208 | 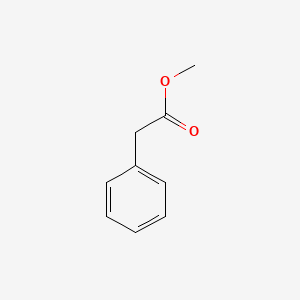 |
0.639 | D0P2GK | 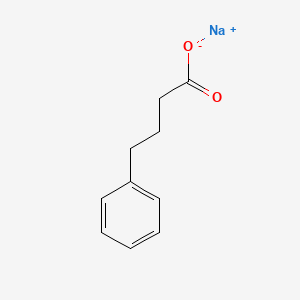 |
0.500 | ||
| ENC004716 | 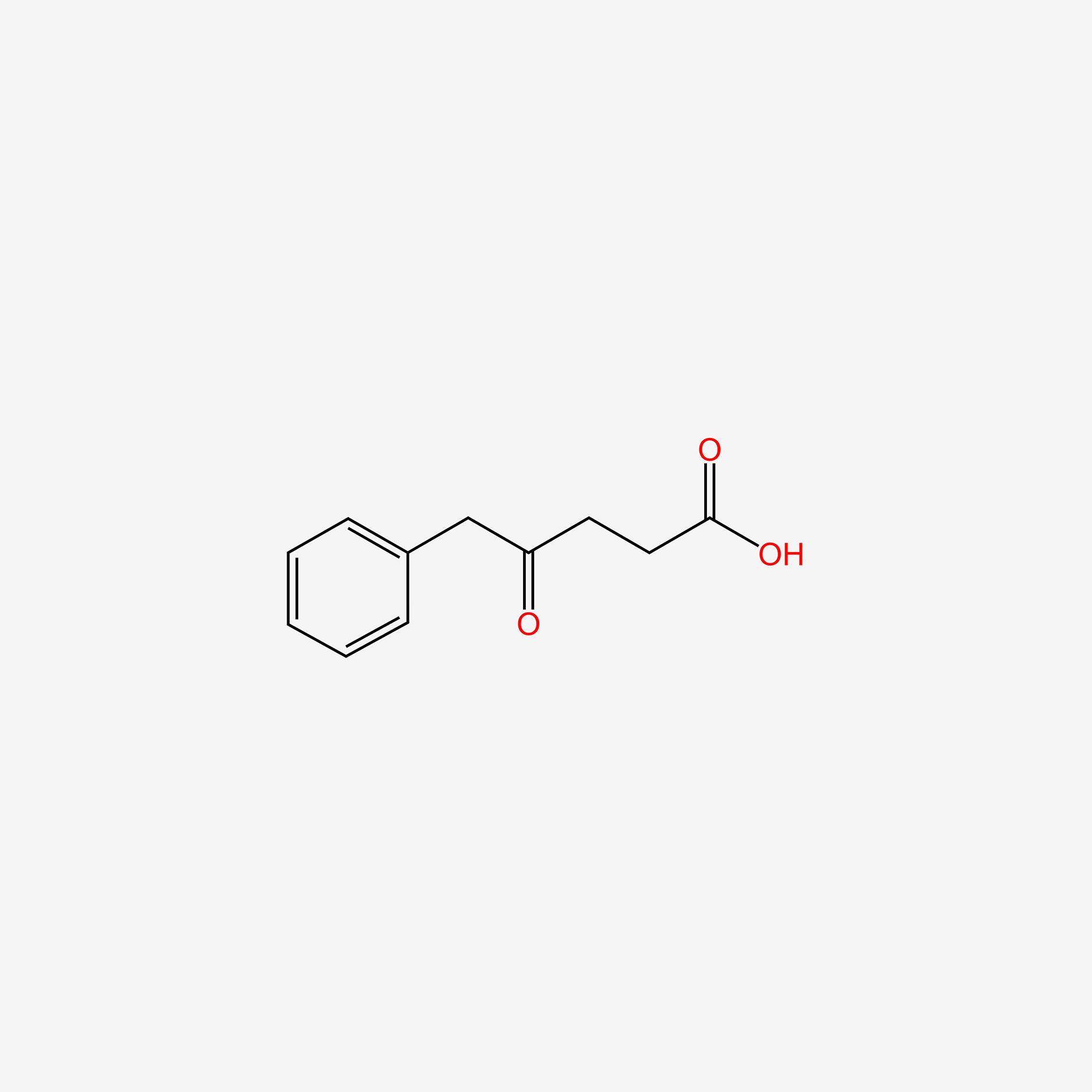 |
0.634 | D00DZN |  |
0.477 | ||
| ENC002014 | 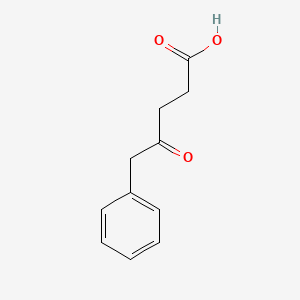 |
0.634 | D01ZJK |  |
0.475 | ||
| ENC000130 | 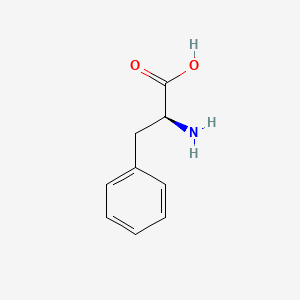 |
0.605 | D0T3LF | 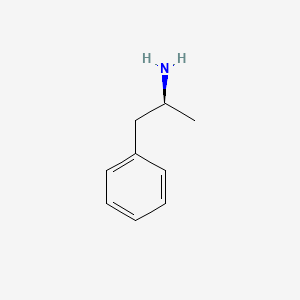 |
0.474 | ||
| ENC000014 |  |
0.594 | D05BMG |  |
0.474 | ||
| ENC001819 |  |
0.564 | D0X9RY |  |
0.472 | ||