NPs Basic Information
|
Name |
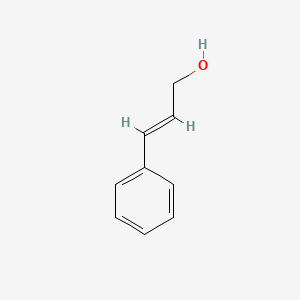
Cinnamyl alcohol
|
| Molecular Formula | C9H10O | |
| IUPAC Name* |
(E)-3-phenylprop-2-en-1-ol
|
|
| SMILES |
C1=CC=C(C=C1)/C=C/CO
|
|
| InChI |
InChI=1S/C9H10O/c10-8-4-7-9-5-2-1-3-6-9/h1-7,10H,8H2/b7-4+
|
|
| InChIKey |
OOCCDEMITAIZTP-QPJJXVBHSA-N
|
|
| Synonyms |
cinnamyl alcohol; 104-54-1; 3-phenylprop-2-en-1-ol; cinnamic alcohol; 3-PHENYL-2-PROPEN-1-OL; 4407-36-7; (E)-3-phenylprop-2-en-1-ol; (E)-cinnamyl alcohol; Styryl carbinol; trans-cinnamyl alcohol; 3-Phenylallyl alcohol; Zimtalcohol; 2-Propen-1-ol, 3-phenyl-; Styrone; Styryl alcohol; Styrylcarbinol; (E)-3-Phenyl-2-propen-1-ol; (2E)-3-phenylprop-2-en-1-ol; FEMA No. 2294; 3-Phenyl-2-propenol; trans-Cinnamyl-alcohol; CHEBI:33227; Phenyl-2-propen-1-ol; 2-Propen-1-ol, 3-phenyl-, (E)-; SS8YOP444F; gamma-Phenylallyl alcohol; CHEMBL324794; Alkohol skoricovy; 1-Phenylprop-1-en-3-ol; (E)-3-phenyl-prop-2-en-1-ol; NSC-8775; NSC-623440; NSC 8775; 3-Fenyl-2-propen-1-ol; Alkohol skoricovy [Czech]; Cinnamic alcohol (natural); CCRIS 3191; HSDB 5011; .gamma.-Phenylallyl alcohol; 3-Fenyl-2-propen-1-ol [Czech]; EINECS 203-212-3; UNII-SS8YOP444F; Propenoic acid, 3-phenyl-, (trans)-; CHEBI:17177; BRN 1903999; Cinnamylalcohol; cinnamyl-alcohol; 2-Propen-y1-ol, 3-phenyl-; AI3-00949; E-cinnamyl alcohol; E-Cinnamic alcohol; NCGC00166097-01; MFCD00002921; (E)-Cinnam Alcohol; trans cinnamyl alcohol; Cinnamyl alcohol purum; 2-Propen-1-ol, 3-phenyl-, (2E)-; Cinnamyl alcohol, 98%; bmse010256; Epitope ID:150920; EC 203-212-3; WLN: Q2U1R; SCHEMBL39219; 1-06-00-00281 (Beilstein Handbook Reference); CINNAMYL ALCOHOL [MI]; CINNAMYL ALCOHOL [FCC]; CINNAMYL ALCOHOL [FHFI]; CINNAMYL ALCOHOL [INCI]; Trans-3-phenylprop-2-en-1-ol; Cinnamyl alcohol, >=98%, FG; NSC8775; trans-3-phenyl-prop-2-en-1-ol; CINNAMYL ALCOHOL [WHO-DD]; (2E)-3-Phenyl-2-propen-1-ol; DTXSID301314144; HY-Y0078; ZINC1529427; BBL027413; BDBM50310446; Cinnamyl alcohol, analytical standard; NSC623440; s5824; STL146348; AKOS005265255; Cinnamyl alcohol, natural, 96%, FG; 1-PHENYL-3-HYDROXY-1-PROPENE; 3-HYDROXY-1-PHENYLPROP-1-ENE; DB14186; BS-14235; Cinnamyl alcohol, purum, >=97.0% (GC); CS-0008356; EN300-19335; A14481; C02394; E75786; EN300-367325; Cinnamyl alcohol, Vetec(TM) reagent grade, 98%; (E)-3-phenyl-2-propen-1-ol(E)-cinnamyl alcohol; A800999; Q204030; SR-01000944721; J-525010; Q-100037; SR-01000944721-1; Z104473562; CINNAMYL ALCOHOL (CONSTITUENT OF CINNAMOMUM CASSIA BARK) [DSC]; CINNAMYL ALCOHOL (CONSTITUENT OF CINNAMOMUM VERUM BARK) [DSC]
|
|
| CAS | 4407-36-7 | |
| PubChem CID | 5315892 | |
| ChEMBL ID | CHEMBL324794 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.17 | ALogp: | 1.9 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.657 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.318 | MDCK Permeability: | 0.00002140 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.706 |
| 30% Bioavailability (F30%): | 0.929 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.968 | Plasma Protein Binding (PPB): | 76.59% |
| Volume Distribution (VD): | 1.038 | Fu: | 19.90% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.926 | CYP1A2-substrate: | 0.25 |
| CYP2C19-inhibitor: | 0.136 | CYP2C19-substrate: | 0.413 |
| CYP2C9-inhibitor: | 0.039 | CYP2C9-substrate: | 0.857 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.513 |
| CYP3A4-inhibitor: | 0.016 | CYP3A4-substrate: | 0.261 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.27 | Half-life (T1/2): | 0.858 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.053 |
| Drug-inuced Liver Injury (DILI): | 0.61 | AMES Toxicity: | 0.091 |
| Rat Oral Acute Toxicity: | 0.024 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.928 | Carcinogencity: | 0.581 |
| Eye Corrosion: | 0.889 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.042 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001091 | 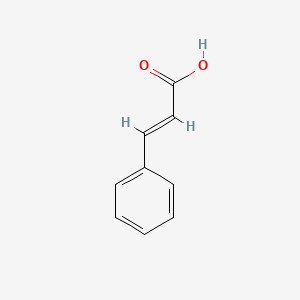 |
0.579 | D01ZJK |  |
0.579 | ||
| ENC001616 | 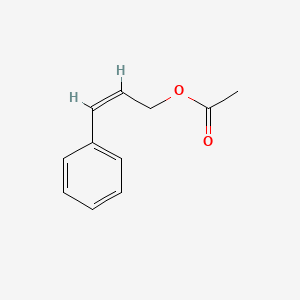 |
0.571 | D05OIS |  |
0.486 | ||
| ENC000023 | 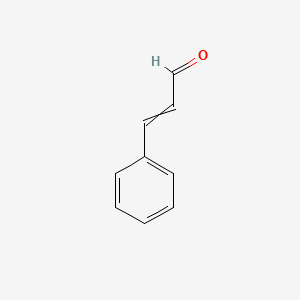 |
0.568 | D0L1WV | 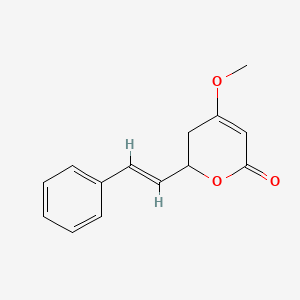 |
0.368 | ||
| ENC000012 | 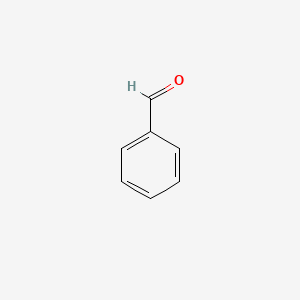 |
0.529 | D0T3LF | 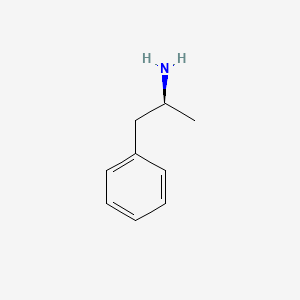 |
0.357 | ||
| ENC000204 | 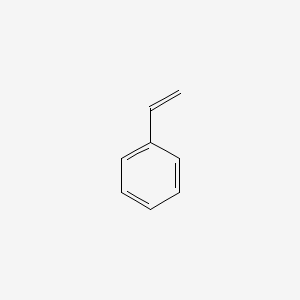 |
0.529 | D05BMG |  |
0.357 | ||
| ENC000014 | 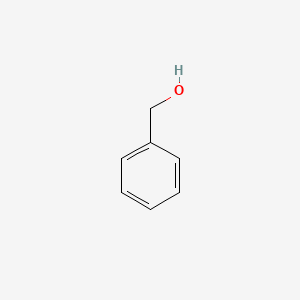 |
0.486 | D0X9RY |  |
0.350 | ||
| ENC001443 | 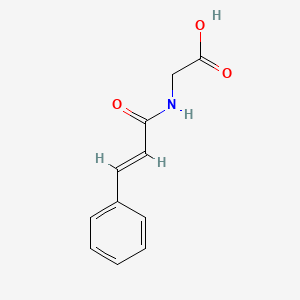 |
0.479 | D0P9AC | 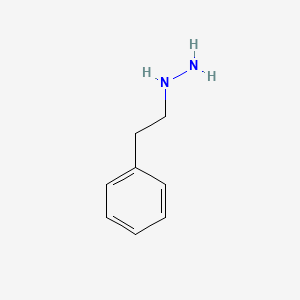 |
0.349 | ||
| ENC001736 | 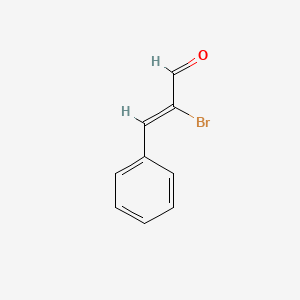 |
0.463 | D0R1CR |  |
0.348 | ||
| ENC000128 | 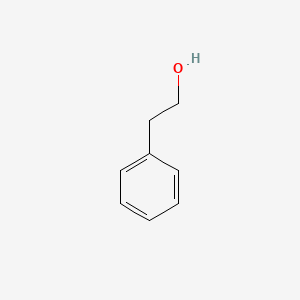 |
0.447 | D00HPK | 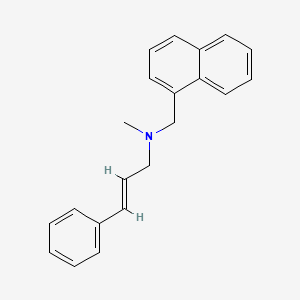 |
0.343 | ||
| ENC000052 | 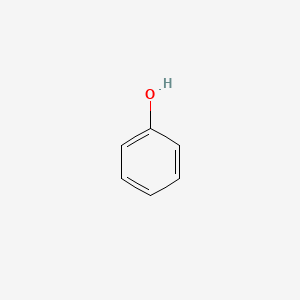 |
0.441 | D0U0RZ | 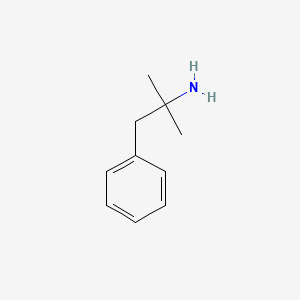 |
0.341 | ||