NPs Basic Information
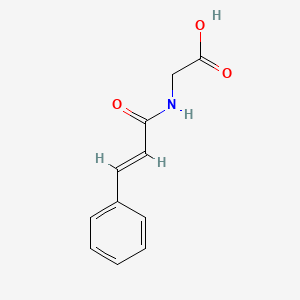
|
Name |
Cinnamoylglycine
|
| Molecular Formula | C11H11NO3 | |
| IUPAC Name* |
2-[[(E)-3-phenylprop-2-enoyl]amino]acetic acid
|
|
| SMILES |
C1=CC=C(C=C1)/C=C/C(=O)NCC(=O)O
|
|
| InChI |
InChI=1S/C11H11NO3/c13-10(12-8-11(14)15)7-6-9-4-2-1-3-5-9/h1-7H,8H2,(H,12,13)(H,14,15)/b7-6+
|
|
| InChIKey |
YAADMLWHGMUGQL-VOTSOKGWSA-N
|
|
| Synonyms |
Cinnamoylglycine; 16534-24-0; N-Cinnamoylglycine; 2-Cinnamamidoacetic acid; N-Cinnamylglycine; 2-[(2E)-3-phenylprop-2-enamido]acetic acid; 62430-40-4; 2-[[(E)-3-phenylprop-2-enoyl]amino]acetic acid; CHEBI:68616; Glycine,N-(1-oxo-3-phenyl-2-propen-1-yl)-; N-[(2E)-3-phenylprop-2-enoyl]glycine; N-(1-oxo-3-phenyl-2-propenyl)-Glycine; N-(1-oxo-3-phenyl-2-propen-1-yl)-Glycine; Glycine, N-(1-oxo-3-phenyl-2-propen-1-yl)-; N-cinnamoyl-Glycine; 2-Cinnamamidoaceticacid; Glycine, N-cinnamoyl-; Glycine, N-cinnamoyl-, E-; CHEMBL456606; SCHEMBL1558162; SCHEMBL9309519; ZINC99385; HMS1447M06; 2-(3-Phenylacrylamido)acetic acid; CS-M1704; STL363041; AKOS000203043; CCG-245338; IDI1_017289; CS-13471; HY-77641; Glycine, N-(1-oxo-3-phenyl-2-propenyl)-; EN300-10753; N-(2-Hydroxy-2-oxoethyl)-3-phenylpropenamide; AB00082962-01; EN300-1448663; N-Cinnamoylglycine, analytical reference material; Q27137048; Z56883153; ECED132A-EF7F-427F-A985-F27822E561C9; C551E21D-9A7D-40AE-83A5-11794CD47554
|
|
| CAS | 16534-24-0 | |
| PubChem CID | 709625 | |
| ChEMBL ID | CHEMBL456606 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 205.21 | ALogp: | 1.2 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 66.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.728 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.886 | MDCK Permeability: | 0.00001970 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.009 |
| Human Intestinal Absorption (HIA): | 0.52 | 20% Bioavailability (F20%): | 0.418 |
| 30% Bioavailability (F30%): | 0.995 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.869 | Plasma Protein Binding (PPB): | 50.09% |
| Volume Distribution (VD): | 0.216 | Fu: | 47.03% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.054 | CYP1A2-substrate: | 0.086 |
| CYP2C19-inhibitor: | 0.053 | CYP2C19-substrate: | 0.058 |
| CYP2C9-inhibitor: | 0.059 | CYP2C9-substrate: | 0.088 |
| CYP2D6-inhibitor: | 0.161 | CYP2D6-substrate: | 0.125 |
| CYP3A4-inhibitor: | 0.011 | CYP3A4-substrate: | 0.151 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.854 | Half-life (T1/2): | 0.858 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.389 |
| Drug-inuced Liver Injury (DILI): | 0.704 | AMES Toxicity: | 0.858 |
| Rat Oral Acute Toxicity: | 0.125 | Maximum Recommended Daily Dose: | 0.021 |
| Skin Sensitization: | 0.786 | Carcinogencity: | 0.18 |
| Eye Corrosion: | 0.307 | Eye Irritation: | 0.956 |
| Respiratory Toxicity: | 0.147 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001091 | 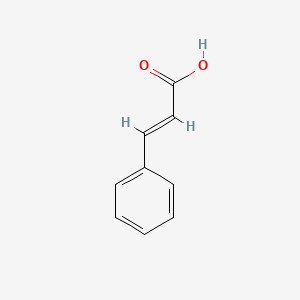 |
0.622 | D01ZJK |  |
0.622 | ||
| ENC004807 |  |
0.515 | D07ONP |  |
0.393 | ||
| ENC001615 | 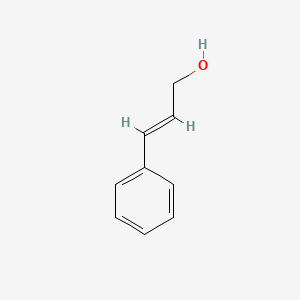 |
0.479 | D03KOZ | 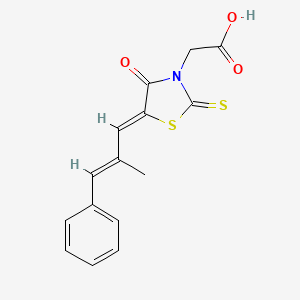 |
0.389 | ||
| ENC001616 | 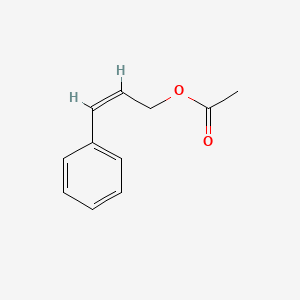 |
0.463 | D0R1CR |  |
0.364 | ||
| ENC000023 | 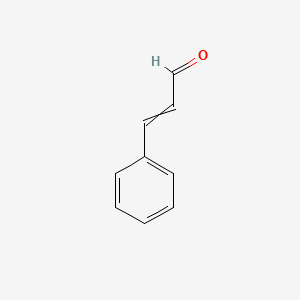 |
0.449 | D0E6OC | 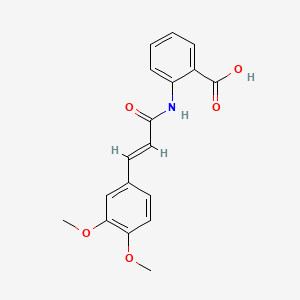 |
0.363 | ||
| ENC000684 | 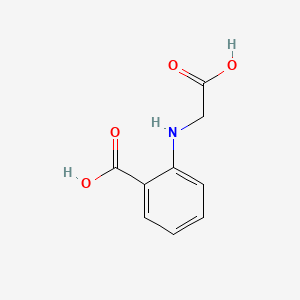 |
0.429 | D0G2MH | 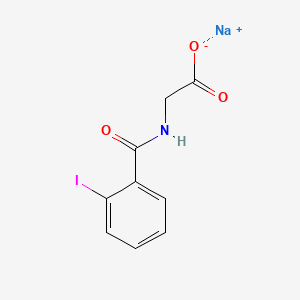 |
0.350 | ||
| ENC000054 | 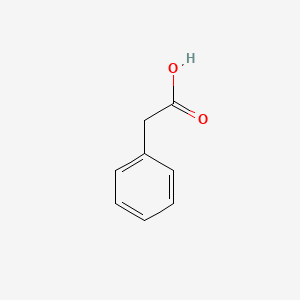 |
0.429 | D0X9RY |  |
0.340 | ||
| ENC001428 | 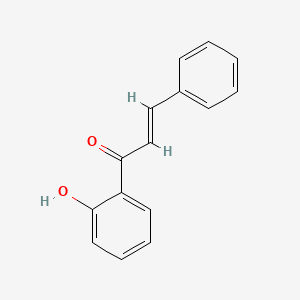 |
0.422 | D00DZN |  |
0.339 | ||
| ENC002014 | 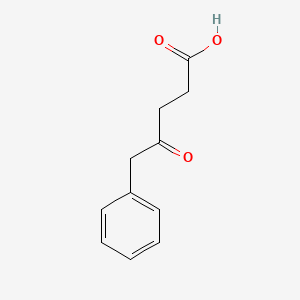 |
0.421 | D05OFX | 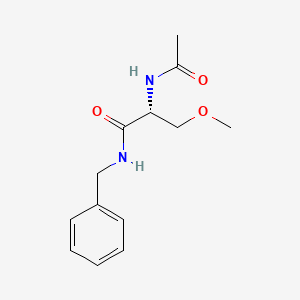 |
0.333 | ||
| ENC004716 | 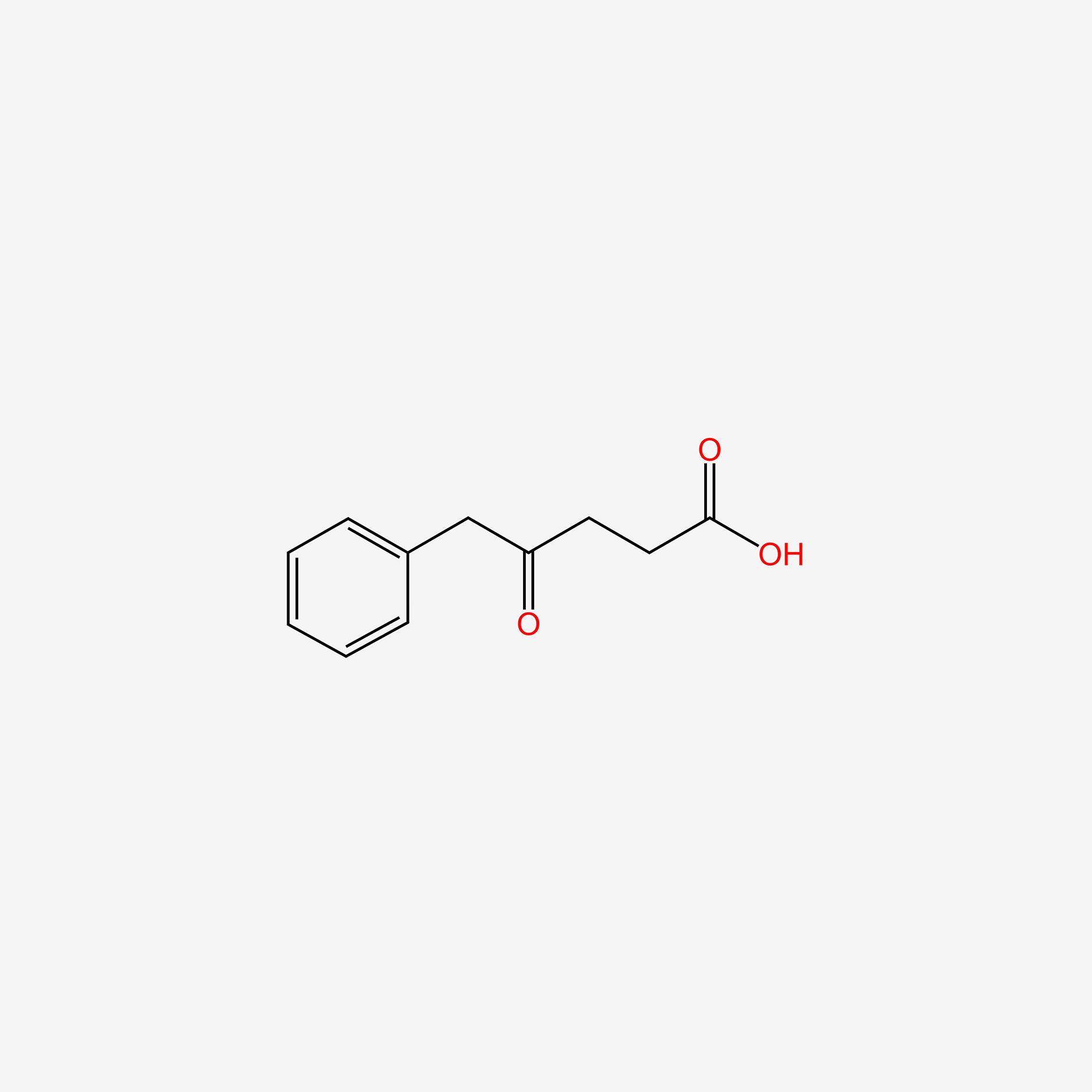 |
0.421 | D06LHG | 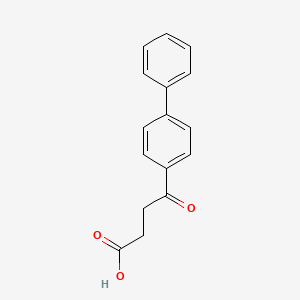 |
0.333 | ||