NPs Basic Information
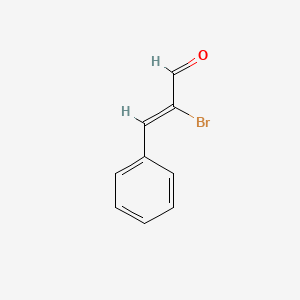
|
Name |
alpha-Bromocinnamaldehyde
|
| Molecular Formula | C9H7BrO | |
| IUPAC Name* |
(Z)-2-bromo-3-phenylprop-2-enal
|
|
| SMILES |
C1=CC=C(C=C1)/C=C(/C=O)\Br
|
|
| InChI |
InChI=1S/C9H7BrO/c10-9(7-11)6-8-4-2-1-3-5-8/h1-7H/b9-6-
|
|
| InChIKey |
WQRWNOKNRHCLHV-TWGQIWQCSA-N
|
|
| Synonyms |
alpha-Bromocinnamaldehyde; 5443-49-2; 2-Bromo-3-phenylacrylaldehyde; Alphabrocine; (Z)-2-bromo-3-phenylprop-2-enal; 2-Bromo-3-phenyl-2-propenal; 33603-90-6; 2-Propenal, 2-bromo-3-phenyl-; B 37; 2-Bromo-3-phenylpropenal; (2Z)-2-bromo-3-phenylprop-2-enal; (Z)-2-bromo-3-phenylacrylaldehyde; Cinnamaldehyde, .alpha.-bromo-; Bromocinnamal; alpha-Bromocinnamic aldehyde; CCRIS 3924; .alpha.-Bromocinnamaldehyde; B 37 (VAN); .alpha.-Bromocinnamic aldehyde; EINECS 226-637-6; NSC 19806; BRN 1099733; MFCD00006965; CINNAMALDEHYDE, alpha-BROMO-; 2-Propenal, 2-bromo-3-phenyl-, (Z)-; alpha -bromocinnamaldehyde; ZINC900; SCHEMBL567062; BCA-XS-150; alpha-Bromocinnamaldehyde, 98%; 99686-39-2; STL281322; (Z)-2-bromo-3-phenyl-prop-2-enal; AKOS000118839; AKOS025310697; (2Z)-2-Bromo-3-phenyl-2-propenal #; AC-11193; LS-13581; B1253; EN300-19519; A830178; EN300-26270395; W-105626; Z2312942099
|
|
| CAS | 5443-49-2 | |
| PubChem CID | 5369403 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 211.05 | ALogp: | 2.7 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.541 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.367 | MDCK Permeability: | 0.00002040 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.029 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.97 | Plasma Protein Binding (PPB): | 96.44% |
| Volume Distribution (VD): | 1.303 | Fu: | 5.58% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.986 | CYP1A2-substrate: | 0.181 |
| CYP2C19-inhibitor: | 0.734 | CYP2C19-substrate: | 0.547 |
| CYP2C9-inhibitor: | 0.277 | CYP2C9-substrate: | 0.1 |
| CYP2D6-inhibitor: | 0.021 | CYP2D6-substrate: | 0.162 |
| CYP3A4-inhibitor: | 0.029 | CYP3A4-substrate: | 0.241 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.032 | Half-life (T1/2): | 0.588 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.003 | Human Hepatotoxicity (H-HT): | 0.886 |
| Drug-inuced Liver Injury (DILI): | 0.816 | AMES Toxicity: | 0.952 |
| Rat Oral Acute Toxicity: | 0.023 | Maximum Recommended Daily Dose: | 0.237 |
| Skin Sensitization: | 0.932 | Carcinogencity: | 0.791 |
| Eye Corrosion: | 0.975 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.963 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000012 | 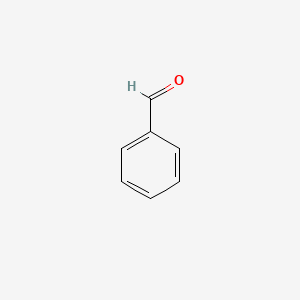 |
0.588 | D01ZJK |  |
0.512 | ||
| ENC000023 | 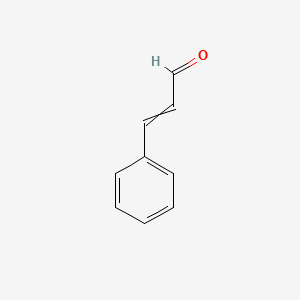 |
0.538 | D0X9RY |  |
0.400 | ||
| ENC001091 | 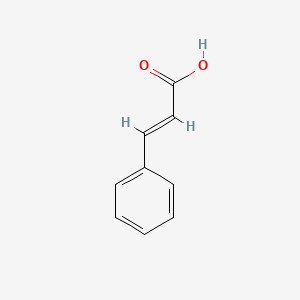 |
0.512 | D05OIS |  |
0.350 | ||
| ENC000204 | 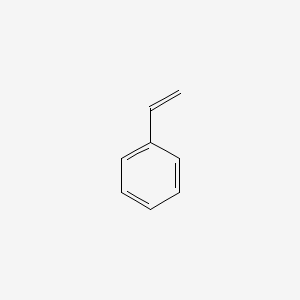 |
0.500 | D03KOZ | 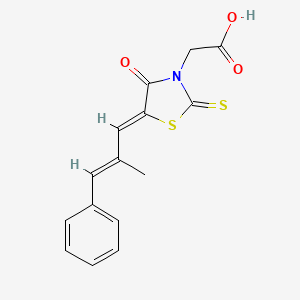 |
0.348 | ||
| ENC001615 | 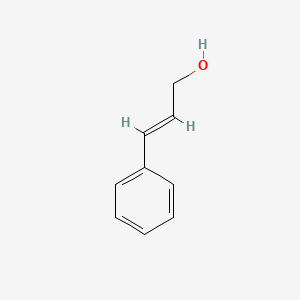 |
0.463 | D0R1CR |  |
0.333 | ||
| ENC001616 | 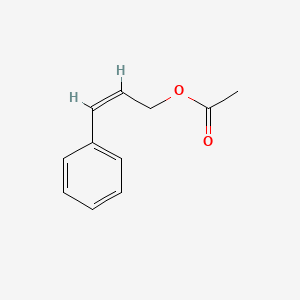 |
0.447 | D0L1WV | 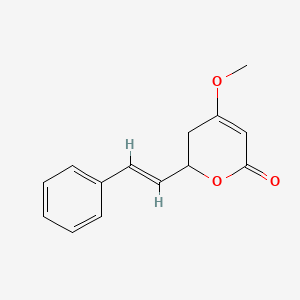 |
0.333 | ||
| ENC000053 | 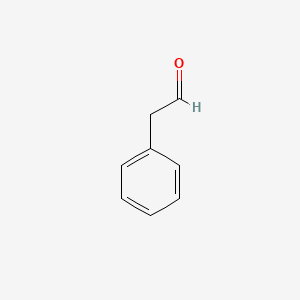 |
0.425 | D0P2GK | 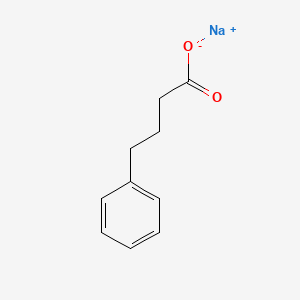 |
0.320 | ||
| ENC001443 | 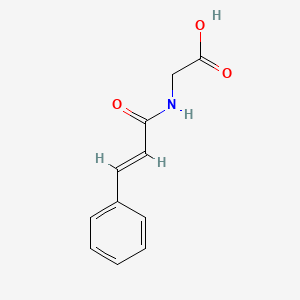 |
0.404 | D07ONP | 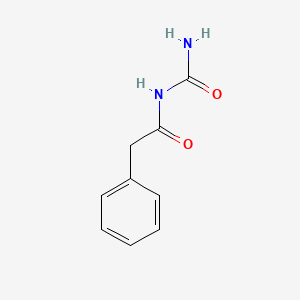 |
0.314 | ||
| ENC000192 | 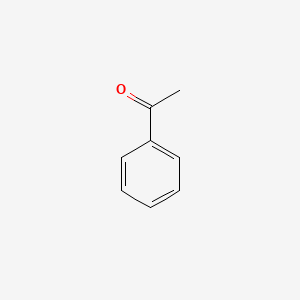 |
0.400 | D0H0HJ | 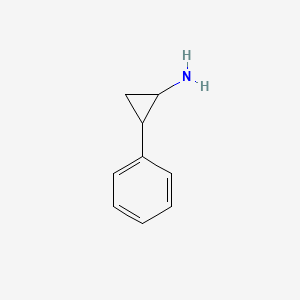 |
0.311 | ||
| ENC000013 | 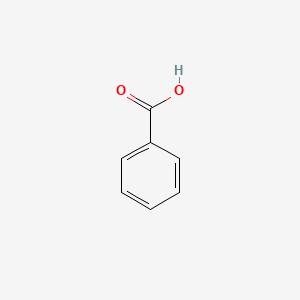 |
0.400 | D05BMG |  |
0.311 | ||