NPs Basic Information
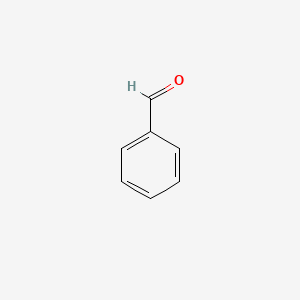
|
Name |
Benzaldehyde
|
| Molecular Formula | C7H6O | |
| IUPAC Name* |
benzaldehyde
|
|
| SMILES |
C1=CC=C(C=C1)C=O
|
|
| InChI |
InChI=1S/C7H6O/c8-6-7-4-2-1-3-5-7/h1-6H
|
|
| InChIKey |
HUMNYLRZRPPJDN-UHFFFAOYSA-N
|
|
| Synonyms |
benzaldehyde; 100-52-7; Benzoic aldehyde; Phenylmethanal; Benzenecarboxaldehyde; Benzenecarbonal; Benzenemethylal; Benzaldehyde FFC; Benzene carbaldehyde; Benzene carboxaldehyde; NCI-C56133; FEMA No. 2127; benzanoaldehyde; Benzylaldehyde; Benzoic acid aldehyde; Benzoyl hydride; Caswell No. 076; Phenylformaldehyde; Benzadehyde; Benzyaldehyde; NSC 7917; Benzaldehyde [NF]; NSC-7917; MFCD00003299; CHEMBL15972; TA269SD04T; CHEBI:17169; Phenylmethanal benzenecarboxaldehyde; Benzaldehyde (NF); NCGC00091819-01; NCGC00091819-02; DSSTox_CID_134; Benzaldehyde, methyl-; DSSTox_RID_79432; DSSTox_GSID_39241; benzaldehyd; Benzaldehyde (natural); Benzaldhyde; BDBM50139371; CAS-100-52-7; CCRIS 2376; HSDB 388; EINECS 202-860-4; UN1990; EPA Pesticide Chemical Code 008601; benzaidehyde; benzaldehvde; benzaldehye; benzaldeyde; UNII-TA269SD04T; phenyl-methanone; AI3-09931; Benzene methylal; Aromatic aldehyde; Benzoylwasserstoff; (phenyl)methanone; Benzaldehyde,(S); PhCHO; Natural Benzaldehyde; 55279-75-9; 2vj1; Benzaldehyde-[13C6]; WLN: VHR; BENZALDEHYDE [II]; BENZALDEHYDE [MI]; SCHEMBL573; BENZALDEHYDE [FCC]; BENZALDEHYDE [FHFI]; BENZALDEHYDE [HSDB]; BENZALDEHYDE [INCI]; EC 202-860-4; BENZALDEHYDE [VANDF]; BENZALDEHYDE [MART.]; ghl.PD_Mitscher_leg0.170; BENZALDEHYDE [USP-RS]; Benzaldehyde, AR, >=99%; Benzaldehyde, LR, >=99%; BIDD:ER0249; DTXSID8039241; BDBM60953; Benzaldehyde, analytical standard; NSC7917; Ald3-H_000012; ZINC895145; BENZALDEHYDE [USP IMPURITY]; Benzaldehyde, >=98%, FG, FCC; Ald3.1-H_000160; Ald3.1-H_000479; Ald3.1-H_000798; Tox21_113069; Tox21_113244; Tox21_200634; MFCD00801585; s5574; STL194067; Benzaldehyde, for synthesis, 95.0%; AKOS000119172; Benzaldehyde [UN1990] [Class 9]; CCG-266041; NA 1989; Benzaldehyde, purum, >=98.0% (GC); Benzaldehyde, ReagentPlus(R), >=99%; NCGC00091819-03; NCGC00258188-01; 8013-76-1; FENTANYL IMPURITY E [EP IMPURITY]; PS-11959; Benzaldehyde, natural, >=98%, FCC, FG; DB-023673; B2379; Benzaldehyde, SAJ special grade, >=98.0%; FT-0622622; FT-0622626; TRIBENOSIDE IMPURITY C [EP IMPURITY]; Benzaldehyde, Vetec(TM) reagent grade, 98%; Benzaldehyde 1000 microg/mL in Dichloromethane; Benzaldehyde, puriss. p.a., >=99.0% (GC); BENZYL ALCOHOL IMPURITY A [EP IMPURITY]; C00193; C00261; D02314; A800226; FENTANYL CITRATE IMPURITY E [EP IMPURITY]; Q372524; SR-01000944375; AMFETAMINE SULFATE IMPURITY D [EP IMPURITY]; Benzaldehyde, purified by redistillation, >=99.5%; SR-01000944375-1; BENZALKONIUM CHLORIDE IMPURITY B [EP IMPURITY]; GLYCOPYRRONIUM BROMIDE IMPURITY F [EP IMPURITY]; F1294-0144; HYDROUS BENZOYL PEROXIDE IMPURITY A [EP IMPURITY]; BENZOYLL PEROXIDE HYDROUS IMPURITY A [EP IMPURITY]; Benzaldehyde, European Pharmacopoeia (EP) Reference Standard; Benzaldehyde, United States Pharmacopeia (USP) Reference Standard; Benzaldehyde, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 100-52-7 | |
| PubChem CID | 240 | |
| ChEMBL ID | CHEMBL15972 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 106.12 | ALogp: | 1.5 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.5 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.306 | MDCK Permeability: | 0.00002160 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.014 | 20% Bioavailability (F20%): | 0.091 |
| 30% Bioavailability (F30%): | 0.016 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.974 | Plasma Protein Binding (PPB): | 75.02% |
| Volume Distribution (VD): | 1.332 | Fu: | 20.42% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.918 | CYP1A2-substrate: | 0.131 |
| CYP2C19-inhibitor: | 0.186 | CYP2C19-substrate: | 0.405 |
| CYP2C9-inhibitor: | 0.057 | CYP2C9-substrate: | 0.33 |
| CYP2D6-inhibitor: | 0.005 | CYP2D6-substrate: | 0.319 |
| CYP3A4-inhibitor: | 0.007 | CYP3A4-substrate: | 0.219 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.971 | Half-life (T1/2): | 0.777 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.052 | Human Hepatotoxicity (H-HT): | 0.024 |
| Drug-inuced Liver Injury (DILI): | 0.038 | AMES Toxicity: | 0.049 |
| Rat Oral Acute Toxicity: | 0.011 | Maximum Recommended Daily Dose: | 0.02 |
| Skin Sensitization: | 0.423 | Carcinogencity: | 0.058 |
| Eye Corrosion: | 0.989 | Eye Irritation: | 0.997 |
| Respiratory Toxicity: | 0.98 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000204 | 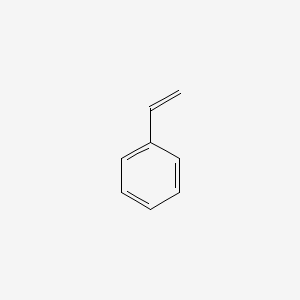 |
0.643 | D01ZJK |  |
0.543 | ||
| ENC000023 | 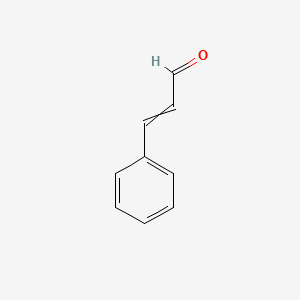 |
0.625 | D0X9RY |  |
0.455 | ||
| ENC001736 | 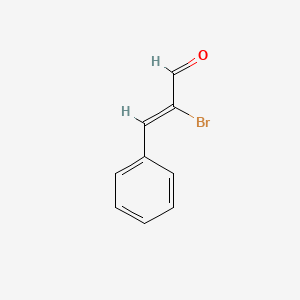 |
0.588 | D05OIS |  |
0.438 | ||
| ENC001091 |  |
0.543 | D0T3LF |  |
0.378 | ||
| ENC000053 |  |
0.531 | D05BMG |  |
0.378 | ||
| ENC001615 |  |
0.529 | D0H0HJ | 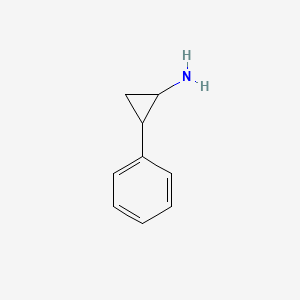 |
0.378 | ||
| ENC000064 | 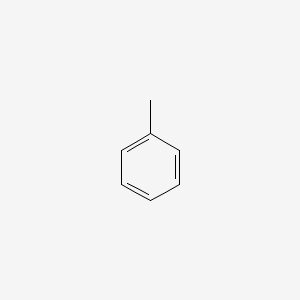 |
0.483 | D0P9AC | 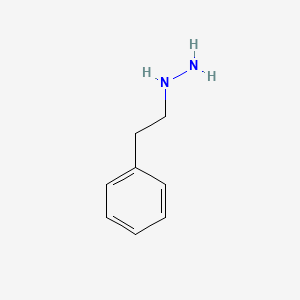 |
0.368 | ||
| ENC000052 | 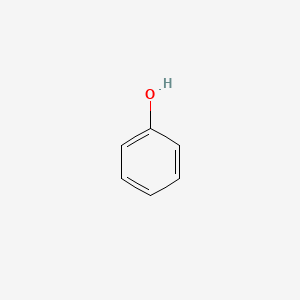 |
0.483 | D0R1CR |  |
0.366 | ||
| ENC001616 | 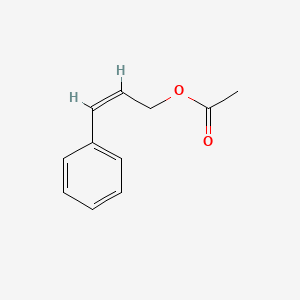 |
0.463 | D0U0RZ | 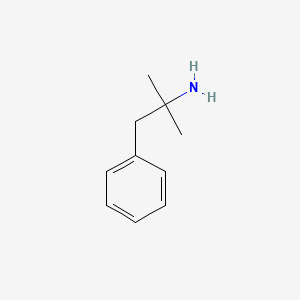 |
0.359 | ||
| ENC000192 | 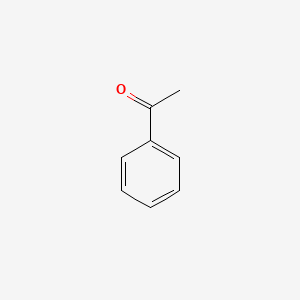 |
0.455 | D0L1WV | 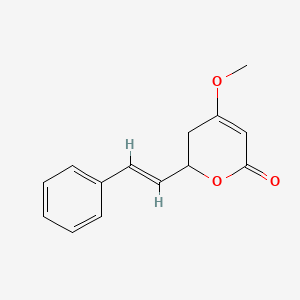 |
0.358 | ||