| Synonyms |
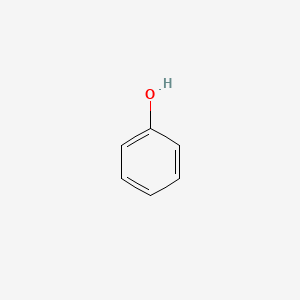
phenol; 108-95-2; carbolic acid; Hydroxybenzene; Phenic acid; Oxybenzene; Phenylic acid; Phenylic alcohol; Benzenol; Monophenol; Phenyl hydrate; Phenyl hydroxide; PhOH; Monohydroxybenzene; Phenyl alcohol; Paoscle; Phenole; Izal; Phenol alcohol; Phenol, liquefied; Acide carbolique; Phenosmolin; Fenolo; Benzene, hydroxy-; Carbolsaure; Fenosmolin; Fenosmoline; Fenol; Liquid phenol; Liquefied phenol; Phenol, pure; Fenolo [Italian]; Phenole [German]; Rcra waste number U188; Campho-Phenique Gel; Phenol [JAN]; Phenic; Carbolsaure [German]; Campho-Phenique Liquid; NCI-C50124; Liquified Phenol; Phenol, molten; Baker's P & S liquid & Ointment; Carbolicum acidum; Fenol [Dutch, Polish]; Baker's P and S Liquid and Ointment; Monohydroxy benzene; Phenol, sulfurated; UN 2312 (molten); Acide carbolique [French]; UN 1671 (solid); NSC 36808; Campho-Phenique Cold Sore Gel; Anbesol; Phenic alcohol; Synthetic phenol; 2-allphenol; Phenol, dimer; RCRA waste no. U188; Phenol, liquified; MFCD00002143; UN1671; UN2312; UN2821; AI3-01814; NSC-36808; CHEMBL14060; 339NCG44TV; DTXSID5021124; CHEBI:15882; ENT-1814; 27073-41-2; Phenol, solid [UN1671] [Poison]; Phenol, molten [UN2312] [Poison]; NCGC00091454-04; DSSTox_CID_1124; Phenol, >=99.0%; DSSTox_RID_75955; DSSTox_GSID_21124; 17442-59-0; 61788-41-8; Caswell No. 649; phenylalcohol; hydroxy benzene; Phenol 100 microg/mL in Methanol; Phenol, liquid; Phenol, solid; Baker's p and s; CAS-108-95-2; CCRIS 504; FEMA No. 3223; HSDB 113; (14C)Phenol; Phenol [USP:JAN]; PHENOL (2,3,4,5,6-D5); EINECS 203-632-7; EPA Pesticide Chemical Code 064001; arenols; UNII-339NCG44TV; Benzophenol; Carbolsaeure; Karbolsaeure; acide phenique; Hydroxy-benzene; Phenol liquid; Phenol molten; Phenol synthetic; Phenol,liquified; Pandy's reagent; Cepastat lozenges; Phenol, labeled with carbon-14; Phenol (liquid); 2-phenyl alcohol; Phenol, synthetic; Phenol, ultrapure; Phenol ACS grade; EINECS 262-972-4; Paoscle (TN); Carbolic acid liquid; Phenol (TN); Phenol,(S); Phenol, ACS reagent; Carbolic acid, liquid; 1ai7; 1li2; 4i7l; Liquefied phenol (TN); PHENOL [VANDF]; PHENOL [FHFI]; PHENOL [HSDB]; PHENOL [IARC]; PHENOL [INCI]; Phenol (JP17/USP); PHENOL [USP-RS]; PHENOL [WHO-DD]; Phenol, detached crystals; PHENOL [II]; PHENOL [MI]; Phenol, >=99%; PHENOL [MART.]; WLN: QR; Liquefied phenol (JP17); bmse000290; bmse010026; C6H5OH; Fenol(DUTCH, POLISH); EC 203-632-7; PHENOL, 80% in ethanol; Phenol, LR, >=99%; 63496-48-0; 65996-83-0; MLS001065591; Phenol, for molecular biology; BIDD:ER0293; PHENOL [EP MONOGRAPH]; Phenol for disinfection (TN); Phenol, natural, 97%, FG; PHENOL [USP MONOGRAPH]; Cuticura pain relieving ointment; CARBOLICUM ACIDUM [HPUS]; Phenol, AR, >=99.5%; PHENOL,LIQUIFIED [VANDF]; BDBM26187; CHEBI:33853; Phenol for disinfection (JP17); 3f39; Phenol 10 microg/mL in Methanol; NSC36808; ZINC5133329; Phenol, Glass Distilled Under Argon; Tox21_113463; Tox21_201639; Tox21_300042; Phenol 5000 microg/mL in Methanol; phenol;phenol [jan];phenol, pure;phenol phenol [jan] phenol, pure; STL194294; AKOS000119025; Tox21_113463_1; DB03255; NA 2821; Phenol, BioXtra, >=99.5% (GC); Phenol, SAJ first grade, >=98.0%; UN 1671; UN 2312; UN 2821; NCGC00091454-01; NCGC00091454-02; NCGC00091454-03; NCGC00091454-05; NCGC00091454-06; NCGC00091454-07; NCGC00254019-01; NCGC00259188-01; Phenol, JIS special grade, >=99.0%; 73607-76-8; AM802906; BP-30160; METHYL SALICYLATE IMPURITY B [EP]; SMR000568492; Phenol 1000 microg/mL in Dichloromethane; Phenol, PESTANAL(R), analytical standard; Liquified Phenol (contains 7-10 % water); METACRESOL IMPURITY A [EP IMPURITY]; FT-0645154; FT-0673707; FT-0693833; P1610; P2771; EN300-19432; C00146; D00033; Phenol, unstabilized, ReagentPlus(R), >=99%; SALICYLIC ACID IMPURITY C [EP IMPURITY]; HEXYLRESORCINOL IMPURITY A [EP IMPURITY]; Phenol, p.a., ACS reagent, 99.5-100.5%; Q130336; J-610001; Phenol, for molecular biology, ~90% (T), liquid; F1908-0106; Phenol, unstabilized, purified by redistillation, >=99%; Z104473830; Phenol, BioUltra, for molecular biology, >=99.5% (GC); Phenol, United States Pharmacopeia (USP) Reference Standard; Liquified Phenol, meets USP testing specifications, >=89.0%; Phenol, BioUltra, for molecular biology, TE-saturated, ~73% (T); Phenol, puriss. p.a., ACS reagent, reag. Ph. Eur., 99.0-100.5%; Phenol, contains hypophosphorous as stabilizer, loose crystals, ACS reagent, >=99.0%; Phenol, puriss., meets analytical specification of Ph. Eur., BP, USP, 99.5-100.5% (GC); Phenol, puriss., meets analytical specification of Ph. Eur., BP, USP, >=99.5% (GC), crystalline (detached)
|