NPs Basic Information
|
Name |
Apigenin
|
| Molecular Formula | C15H10O5 | |
| IUPAC Name* |
5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
|
| SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O
|
|
| InChI |
InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
|
|
| InChIKey |
KZNIFHPLKGYRTM-UHFFFAOYSA-N
|
|
| Synonyms |
apigenin; 520-36-5; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; Chamomile; Versulin; 4',5,7-Trihydroxyflavone; Apigenol; Apigenine; Spigenin; C.I. Natural Yellow 1; 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one; 5,7,4'-Trihydroxyflavone; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; Pelargidenon 1449; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone; NSC 83244; 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-; UCCF 031; FLAVONE, 4',5,7-TRIHYDROXY-; CHEBI:18388; CHEMBL28; MFCD00006831; NSC-83244; 8002-66-2; 7V515PI7F6; NSC83244; CAS-520-36-5; DSSTox_CID_2391; DSSTox_RID_76568; DSSTox_GSID_22391; 4',5,7-Trihydroxyflavone;Apigenol;C.I. Natural Yellow 1; SMR000326850; CCRIS 3789; 4′,5,7-Trihydroxyflavone; SR-01000075663; EINECS 208-292-3; BRN 0262620; Pelargidenone; UNII-7V515PI7F6; Chamomile Powder; HSDB 7573; 4der; 4dgm; 4hkk; Naringenin, 18; Prestwick_719; Apigenin, 13; APEGENIN; Tocris-1227; 3cf9; ST056301; APIGENIN [HSDB]; APIGENIN [INCI]; 4',7-Trihydroxyflavone; APIGENIN [MI]; BiomolKI_000078; Prestwick0_000414; Prestwick1_000414; Prestwick2_000414; Prestwick3_000414; Spectrum2_000428; Spectrum3_001882; Spectrum4_001999; Lopac-A-3145; APIGENIN [USP-RS]; APIGENIN [WHO-DD]; BiomolKI2_000082; 4,5, 7-Trihydroxyflavone; PELARGIDENON-1449; CI NATURAL YELLOW 1; Lopac0_000065; Oprea1_622293; SCHEMBL19428; 4',5,7-trihydroxy-Flavone; Apigenin, analytical standard; BSPBio_000368; BSPBio_003384; KBioGR_002565; SPECTRUM200846; 5-18-04-00574 (Beilstein Handbook Reference); MLS000697626; MLS000859991; MLS001074874; MLS006011839; BIDD:ER0135; DivK1c_000798; SCHEMBL222227; SPBio_000416; SPBio_002307; ghl.PD_Mitscher_leg0.1194; BDBM7458; BPBio1_000406; GTPL4136; MEGxp0_000176; UCCF-031; DTXSID6022391; ACon1_002450; cid_5280443; HMS502H20; KBio1_000798; KBio3_002887; NINDS_000798; Bio1_000376; Bio1_000865; Bio1_001354; HMS1569C10; HMS1922P22; HMS2096C10; HMS2230D17; HMS3260M11; HMS3267D21; HMS3373B18; HMS3412A08; HMS3561P09; HMS3655D18; HMS3676A08; HMS3866D03; Apigenin, >=95.0% (HPLC); 4',5,7-Trihydroxyflavone, 97%; BCP28288; HY-N1201; ZINC3871576; Tox21_201542; Tox21_302884; Tox21_500065; Apigenin; 4',5,7-Trihydroxyflavone; BBL010499; CCG-40061; HB0117; HSCI1_000221; LMPK12110005; NSC815095; s2262; STK801630; ZB1873; AKOS002140699; AC-8011; CS-5432; DB07352; LP00065; ND-9076; NSC-815095; SDCCGMLS-0066379.P001; SDCCGSBI-0050053.P003; IDI1_000798; SMP2_000338; Apigenin, >=97% (TLC), from citrus; NCGC00015049-01; NCGC00015049-02; NCGC00015049-03; NCGC00015049-04; NCGC00015049-05; NCGC00015049-06; NCGC00015049-07; NCGC00015049-08; NCGC00015049-09; NCGC00015049-10; NCGC00015049-11; NCGC00015049-12; NCGC00015049-13; NCGC00015049-14; NCGC00015049-15; NCGC00015049-16; NCGC00015049-18; NCGC00015049-28; NCGC00025057-01; NCGC00025057-02; NCGC00025057-03; NCGC00025057-04; NCGC00025057-05; NCGC00025057-06; NCGC00025057-07; NCGC00025057-08; NCGC00025057-09; NCGC00169835-01; NCGC00169835-02; NCGC00169835-03; NCGC00256419-01; NCGC00259092-01; NCGC00260750-01; LY080400; NCI60_041830; SY005957; TS-00897; LY 080400; LY-080400; EU-0100065; FT-0622445; FT-0623582; FT-0662251; SW196866-2; 20A365; A 3145; C01477; K00045; O11338; Apigenin 100 microg/mL in Acetonitrile:Methanol; Apigenin, >=97% (TLC), from parsley, powder; Biochem Biophys Res Comm 212: 767 (1997); EN300-7382221; 5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one; A828903; APIGENIN (CONSTITUENT OF CHAMOMILE) [DSC]; Apigenin, primary pharmaceutical reference standard; Q424567; 4 inverted exclamation mark ,5,7-trihydroxyflavone; Q-100586; Q-200822; SR-01000075663-1; SR-01000075663-3; SR-01000075663-7; SR-01000075663-8; BRD-K01493881-001-10-4; BRD-K01493881-001-17-9; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #; 4H-1-Benzopyran-4-one,7-dihydroxy-2-(4-hydroxyphenyl)-; D50A2D8A-6D8B-4708-B21E-2DE9580D033F; Apigenin, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 520-36-5 | |
| PubChem CID | 5280443 | |
| ChEMBL ID | CHEMBL28 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 270.24 | ALogp: | 1.7 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 87.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 20 | QED Weighted: | 0.63 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.847 | MDCK Permeability: | 0.00001160 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.82 |
| Human Intestinal Absorption (HIA): | 0.015 | 20% Bioavailability (F20%): | 0.995 |
| 30% Bioavailability (F30%): | 0.999 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.012 | Plasma Protein Binding (PPB): | 97.25% |
| Volume Distribution (VD): | 0.51 | Fu: | 3.67% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.988 | CYP1A2-substrate: | 0.145 |
| CYP2C19-inhibitor: | 0.588 | CYP2C19-substrate: | 0.051 |
| CYP2C9-inhibitor: | 0.602 | CYP2C9-substrate: | 0.939 |
| CYP2D6-inhibitor: | 0.792 | CYP2D6-substrate: | 0.778 |
| CYP3A4-inhibitor: | 0.833 | CYP3A4-substrate: | 0.126 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.022 | Half-life (T1/2): | 0.856 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.057 | Human Hepatotoxicity (H-HT): | 0.072 |
| Drug-inuced Liver Injury (DILI): | 0.854 | AMES Toxicity: | 0.475 |
| Rat Oral Acute Toxicity: | 0.057 | Maximum Recommended Daily Dose: | 0.433 |
| Skin Sensitization: | 0.928 | Carcinogencity: | 0.277 |
| Eye Corrosion: | 0.011 | Eye Irritation: | 0.945 |
| Respiratory Toxicity: | 0.266 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
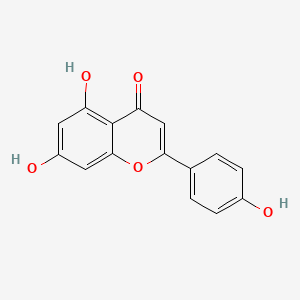
| ENC001534 | 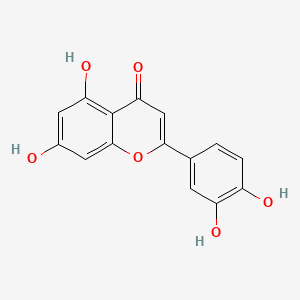 |
0.701 | D04AIT |  |
0.701 | ||
| ENC001573 | 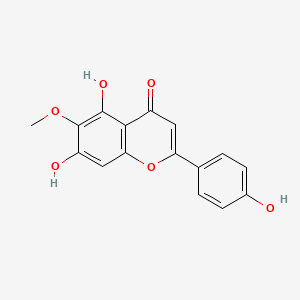 |
0.696 | D0K8KX |  |
0.506 | ||
| ENC001548 |  |
0.676 | D06GCK |  |
0.437 | ||
| ENC001550 | 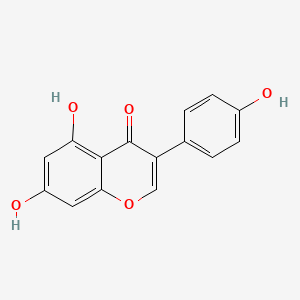 |
0.672 | D04XEG | 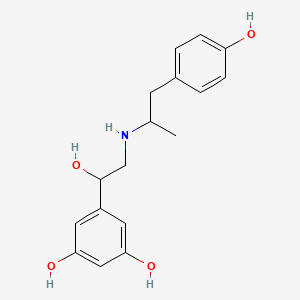 |
0.360 | ||
| ENC001771 | 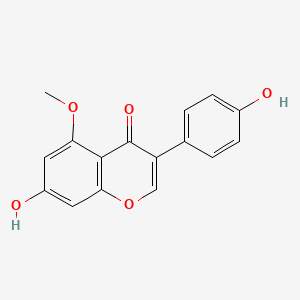 |
0.533 | D07MGA |  |
0.360 | ||
| ENC001529 | 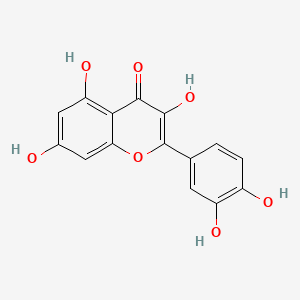 |
0.506 | D06TJJ | 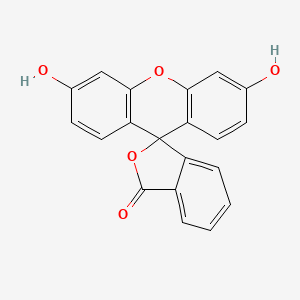 |
0.333 | ||
| ENC001751 | 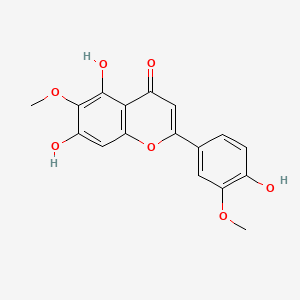 |
0.488 | D03UOT |  |
0.322 | ||
| ENC001576 | 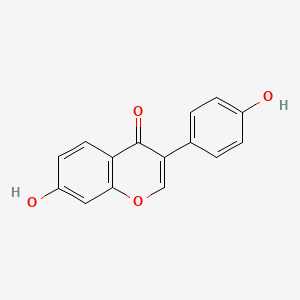 |
0.486 | D0R6BI | 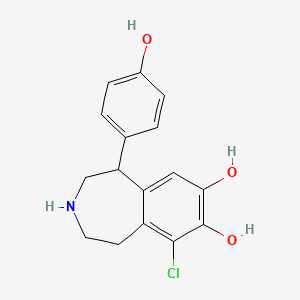 |
0.322 | ||
| ENC001532 | 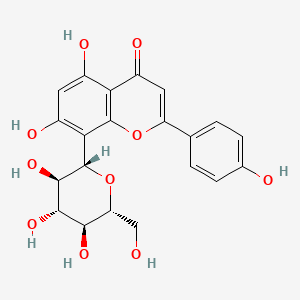 |
0.484 | D08LFZ | 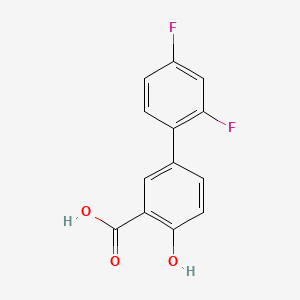 |
0.313 | ||
| ENC002757 | 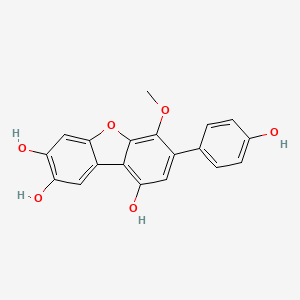 |
0.482 | D0J7RK | 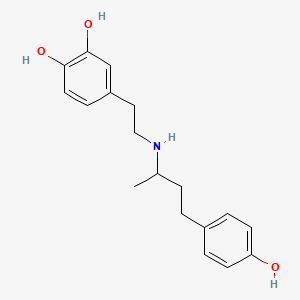 |
0.311 | ||