NPs Basic Information
|
Name |
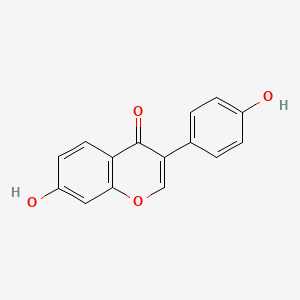
Daidzein
|
| Molecular Formula | C15H10O4 | |
| IUPAC Name* |
7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one
|
|
| SMILES |
C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3)O)O
|
|
| InChI |
InChI=1S/C15H10O4/c16-10-3-1-9(2-4-10)13-8-19-14-7-11(17)5-6-12(14)15(13)18/h1-8,16-17H
|
|
| InChIKey |
ZQSIJRDFPHDXIC-UHFFFAOYSA-N
|
|
| Synonyms |
daidzein; 486-66-8; 4',7-Dihydroxyisoflavone; Daidzeol; 7,4'-Dihydroxyisoflavone; 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one; 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one; 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-; 7-Hydroxy-3-(4-hydroxyphenyl)-4-benzopyrone; C15H10O4; 4,7-Dihydroxyisoflavone; MFCD00016954; CHEMBL8145; d-(+)-alpha-methylbenzylamine; CHEBI:28197; 6287WC5J2L; Daidzein,(S); DSSTox_CID_2310; 7-Hydroxy-3-(4-hydroxy-phenyl)-chromen-4-one; DSSTox_RID_76543; DSSTox_GSID_22310; CAS-486-66-8; SMR000326832; CCRIS 7600; K 251b; SR-01000075492; EINECS 207-635-4; BRN 0231523; 4',7-Dihydroxy-iso-flavone; UNII-6287WC5J2L; Isoflavone, 4',7-dihydroxy-; NPI 031E; Spectrum_000255; Tocris-1417; 7-Hydroxy-3-(4-hydroxy-phenyl)-chromone; DAIDZEIN [INCI]; DAIDZEIN [MI]; BiomolKI_000060; Spectrum2_000053; Spectrum3_000191; Spectrum4_001964; Spectrum5_000857; Lopac-D-7802; DAIDZEIN [MART.]; BiomolKI2_000066; DAIDZEIN [USP-RS]; DAIDZEIN [WHO-DD]; UPCMLD-DP052; 4',7-dihydroxy isoflavone; 7-HYDROXY-3-(4-HYDROXYPHENYL)CHROMONE; 4',7-dihydroxy-Isoflavone; Lopac0_000412; Oprea1_182317; Oprea1_305345; SCHEMBL19814; BSPBio_001741; Daidzein, analytical standard; KBioGR_002432; KBioSS_000735; SPECTRUM200789; 5-18-04-00089 (Beilstein Handbook Reference); MLS000859973; MLS001304056; MLS006011853; BIDD:ER0120; DivK1c_001023; SPBio_000205; Daidzein, >=98%, synthetic; BMK1-F12; GTPL2828; MEGxm0_000123; NPI-031E; d-(+)-alpha-methylbenzyl amine; DTXSID9022310; UPCMLD-DP052:001; ACon0_001477; ACon1_000543; BDBM23420; HMS503M07; K-251b; KBio1_001023; KBio2_000735; KBio2_003303; KBio2_005871; KBio3_001241; 4',7-Dihydroxyisoflavone, 97%; NINDS_001023; 7,4'-Dihydroxy-isoflavone (3a); HMS1922P18; HMS2233H24; HMS3261C06; HMS3267J04; HMS3370C03; HMS3412O12; HMS3468L18; HMS3649B20; HMS3655A18; HMS3676O12; ALBB-015933; AMY25222; BCP28286; HY-N0019; Tox21_201444; Tox21_303650; Tox21_500412; BBL010490; CCG-38357; HB2488; LMPK12050038; s1849; STK801626; ZINC18847034; Daidzein (4',7-Dihydroxyisoflavone); 7-hydroxy-3-(4-hydroxyphenyl)-4H-; AKOS002385052; AC-6035; Daidzein, purum, >=98.0% (TLC); DB13182; KS-5260; LP00412; SDCCGMLS-0066422.P001; SDCCGSBI-0050397.P003; IDI1_001023; SMP1_000089; NCGC00015365-01; NCGC00015365-02; NCGC00015365-03; NCGC00015365-04; NCGC00015365-05; NCGC00015365-06; NCGC00015365-07; NCGC00015365-08; NCGC00015365-09; NCGC00015365-10; NCGC00015365-11; NCGC00015365-12; NCGC00015365-13; NCGC00015365-14; NCGC00015365-15; NCGC00015365-16; NCGC00015365-17; NCGC00015365-18; NCGC00015365-31; NCGC00025156-01; NCGC00025156-02; NCGC00025156-03; NCGC00025156-04; NCGC00025156-05; NCGC00025156-06; NCGC00025156-07; NCGC00025156-08; NCGC00025156-09; NCGC00025156-10; NCGC00168978-01; NCGC00168978-02; NCGC00257367-01; NCGC00258995-01; NCGC00261097-01; SY049259; BB 0259520; D2668; EU-0100412; FT-0603419; FT-0665448; FT-0665449; SW218107-2; 7-hydroxy-3-(4-hydroxyphenyl)-chromen-4-one; D 7802; EN300-116213; S00273; US8552057, 7; AB00052712-08; AB00052712_10; 486D668; DAIDZEIN (CONSTITUENT OF ASTRAGALUS) [DSC]; Daidzein, primary pharmaceutical reference standard; Q408732; DAIDZEIN (CONSTITUENT OF RED CLOVER) [DSC]; Q-200924; SR-01000075492-1; SR-01000075492-3; SR-01000075492-7; SR-01000075492-8; BRD-K42095107-001-02-3; BRD-K42095107-001-05-6; BRD-K42095107-001-08-0; SR-01000075492-12; 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1- benzopyran-4-one; 80E3ED75-D852-4D97-9BD6-B5ADE7EA25A1; DAIDZEIN (CONSTITUENT OF SOY ISOFLAVONES) [DSC]; Z1494829514; Daidzein, United States Pharmacopeia (USP) Reference Standard; Daidzein, Pharmaceutical Secondary Standard; Certified Reference Material; ZF1
|
|
| CAS | 486-66-8 | |
| PubChem CID | 5281708 | |
| ChEMBL ID | CHEMBL8145 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 254.24 | ALogp: | 2.5 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 66.8 | Aromatic Rings: | 3 |
| Heavy Atoms: | 19 | QED Weighted: | 0.695 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.643 | MDCK Permeability: | 0.00001220 |
| Pgp-inhibitor: | 0.006 | Pgp-substrate: | 0.937 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.23 |
| 30% Bioavailability (F30%): | 0.856 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.054 | Plasma Protein Binding (PPB): | 97.08% |
| Volume Distribution (VD): | 0.495 | Fu: | 1.96% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.976 | CYP1A2-substrate: | 0.127 |
| CYP2C19-inhibitor: | 0.808 | CYP2C19-substrate: | 0.054 |
| CYP2C9-inhibitor: | 0.375 | CYP2C9-substrate: | 0.945 |
| CYP2D6-inhibitor: | 0.929 | CYP2D6-substrate: | 0.89 |
| CYP3A4-inhibitor: | 0.908 | CYP3A4-substrate: | 0.173 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.802 | Half-life (T1/2): | 0.846 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.072 | Human Hepatotoxicity (H-HT): | 0.053 |
| Drug-inuced Liver Injury (DILI): | 0.52 | AMES Toxicity: | 0.061 |
| Rat Oral Acute Toxicity: | 0.333 | Maximum Recommended Daily Dose: | 0.188 |
| Skin Sensitization: | 0.895 | Carcinogencity: | 0.617 |
| Eye Corrosion: | 0.019 | Eye Irritation: | 0.97 |
| Respiratory Toxicity: | 0.101 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001550 | 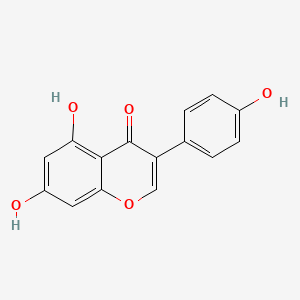 |
0.667 | D0R2OA | 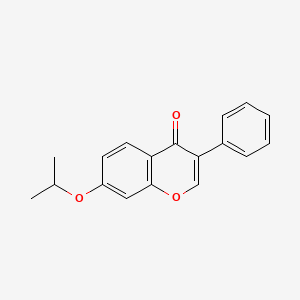 |
0.481 | ||
| ENC001771 | 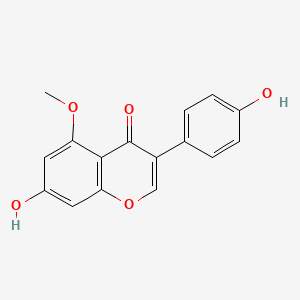 |
0.638 | D06TJJ | 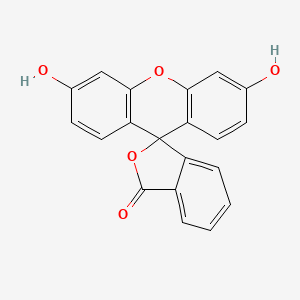 |
0.385 | ||
| ENC003703 | 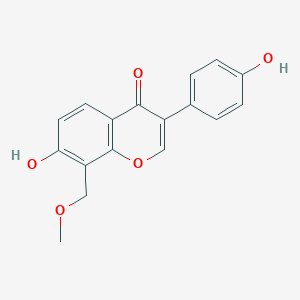 |
0.611 | D03UOT |  |
0.357 | ||
| ENC004476 | 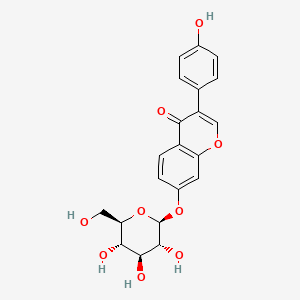 |
0.551 | D01XBA | 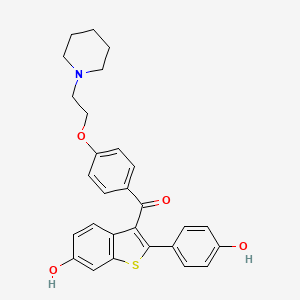 |
0.342 | ||
| ENC001533 | 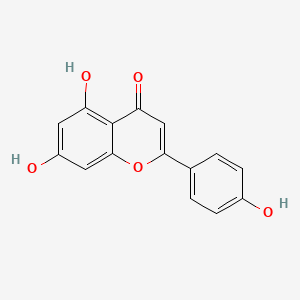 |
0.486 | D0Y2NE | 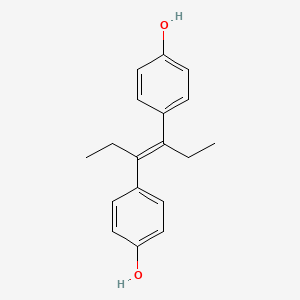 |
0.341 | ||
| ENC001548 | 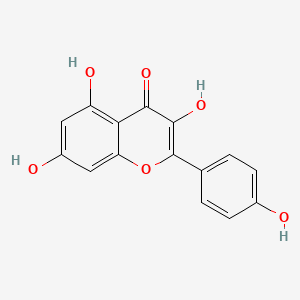 |
0.474 | D09ZQN | 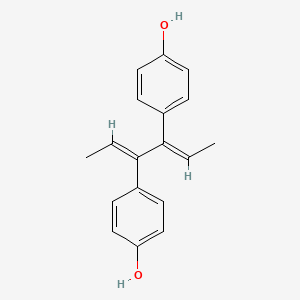 |
0.341 | ||
| ENC002800 | 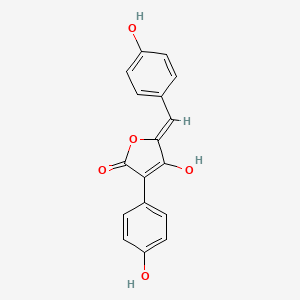 |
0.468 | D04AIT |  |
0.333 | ||
| ENC005038 | 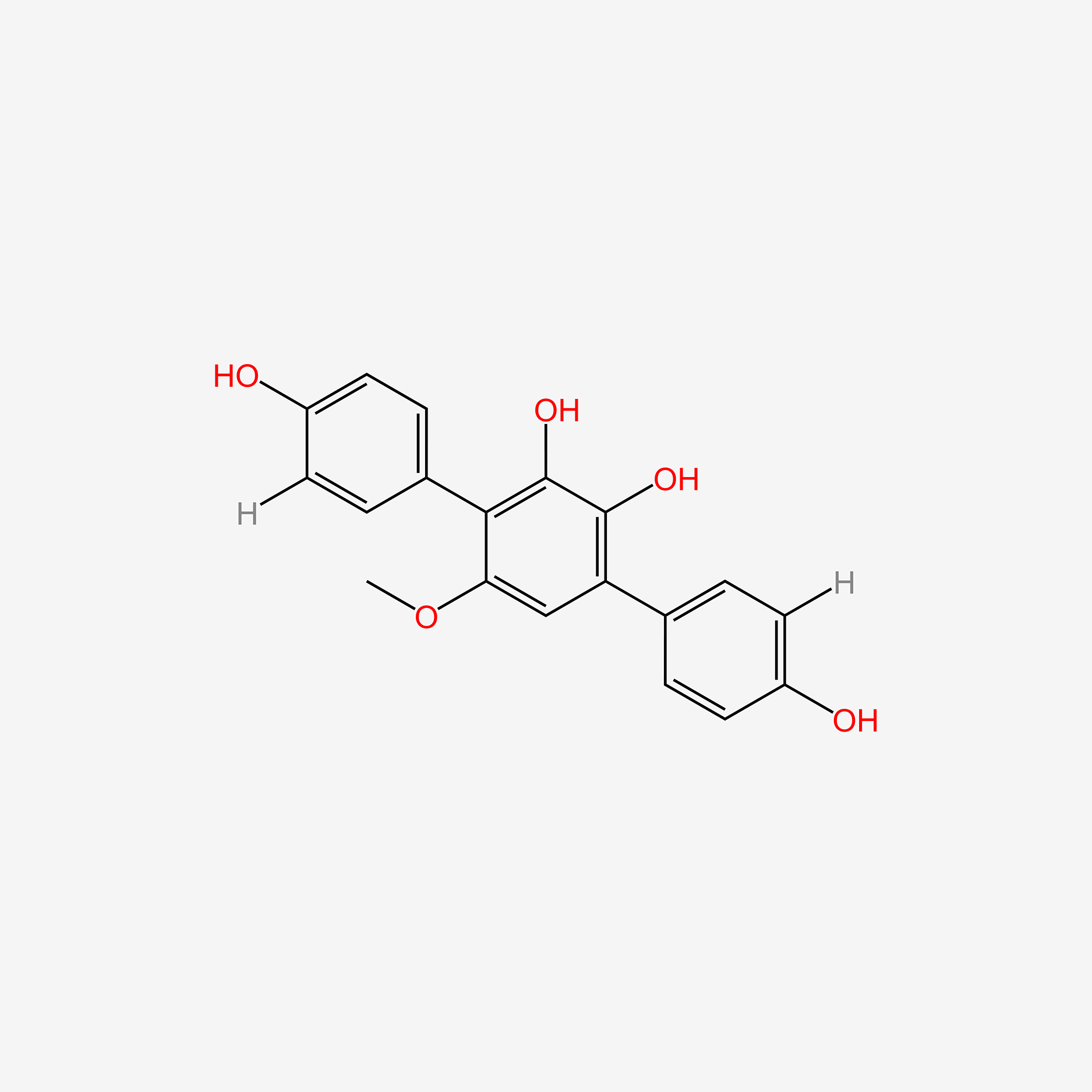 |
0.440 | D00LFB | 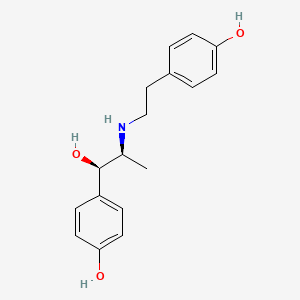 |
0.329 | ||
| ENC002755 | 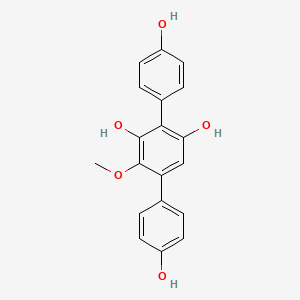 |
0.440 | D0K8KX |  |
0.326 | ||
| ENC004475 | 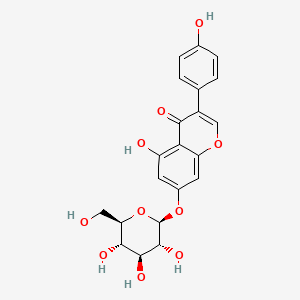 |
0.429 | D0JY8T | 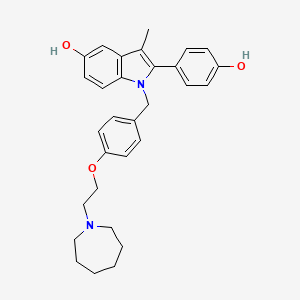 |
0.311 | ||