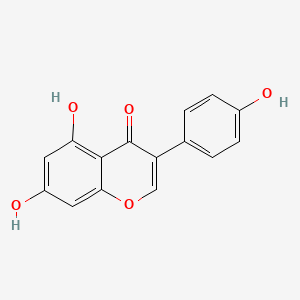
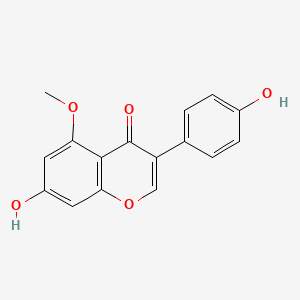
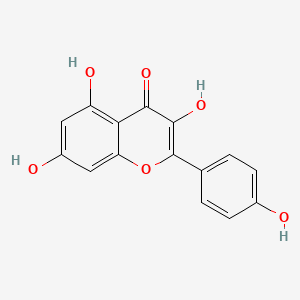
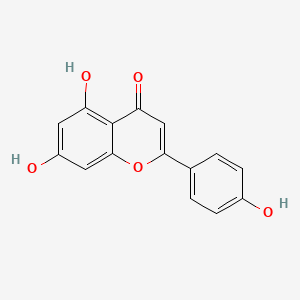
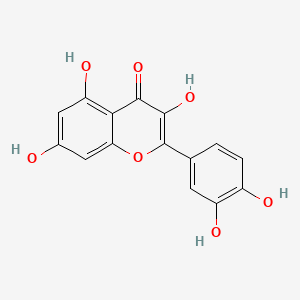
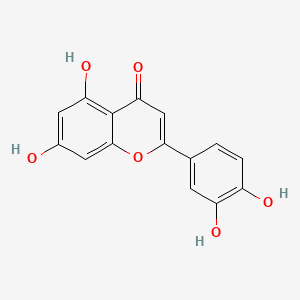
| Synonyms |
genistein; 446-72-0; Prunetol; 4',5,7-Trihydroxyisoflavone; Genisterin; Genisteol; Sophoricol; 5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one; 5,7,4'-Trihydroxyisoflavone; Bonistein; Differenol A; 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one; NPI 031L; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-; C.I. 75610; SIPI 807-1; MFCD00016952; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4-benzopyrone; NSC 36586; Genistein [USAN]; NSC36586; CHEMBL44; NSC-36586; Bio 300; ISOFLAVONE, 4',5,7-TRIHYDROXY-; MLS000738127; DH2M523P0H; NPI-031L; Genestein; CHEBI:28088; BIO-300; SIPI-807-1; Genistein (USAN); FW-635I-2; GEN; NCGC00015479-09; DSSTox_CID_2308; DSSTox_RID_76542; DSSTox_GSID_22308; CAS-446-72-0; Lactoferrin-genistein; CCRIS 7675; 4',5, 7-Trihydroxyisoflavone; SR-01000075498; EINECS 207-174-9; BRN 0263823; UNII-DH2M523P0H; STO514; HSDB 7475; PTI G4660; PTI-G4660; SIPI-9764-I; 3kgt; 3kgu; Genistein, 8; Genistein,(S); PTI-G 4660; TNP00151; Genistein (flavonoid); Spectrum_000320; Tocris-1110; 1x7r; 2qa8; Genistein 85% HPLC; GENISTEIN [INN]; SpecPlus_000305; GENISTEIN [MI]; GENISTEIN [HSDB]; GENISTEIN [INCI]; Spectrum2_000638; Spectrum3_000678; Spectrum4_001543; Spectrum5_000106; Lopac-G-6649; 4',7-Trihydroxyisoflavone; GENISTEIN [MART.]; MolMap_000022; UPCMLD-DP096; G 6649; GENISTEIN [USP-RS]; GENISTEIN [WHO-DD]; 4,5,7-Trihydroxyisoflavone; Isoflavone,5,7-trihydroxy-; Lopac0_000520; Oprea1_224620; Oprea1_437815; SCHEMBL19166; BSPBio_002375; KBioGR_002006; KBioGR_002564; KBioSS_000800; KBioSS_002573; NPI031L; SPECTRUM210296; 5-18-04-00594 (Beilstein Handbook Reference); BIDD:ER0113; DivK1c_006401; Genistein, analytical standard; SPBio_000636; 4',5,7-trihydroxy-Isoflavone; GTPL2826; MEGxp0_000568; 4,5,7-Trihydroxy Iso-Flavone; DTXSID5022308; UPCMLD-DP096:001; ACon1_001065; BDBM19459; cid_5280961; KBio1_001345; KBio2_000800; KBio2_002564; KBio2_003368; KBio2_005132; KBio2_005936; KBio2_007700; KBio3_001595; KBio3_003042; CHEBI: 28088; cMAP_000086; Bio1_000445; Bio1_000934; Bio1_001423; HMS2271K09; HMS3261H21; HMS3267K14; HMS3412I13; HMS3428M01; HMS3649B22; HMS3654D17; HMS3676I13; HMS3742I07; ACT05962; ALBB-015886; AMY25676; BCP07581; Tox21_110161; Tox21_201428; Tox21_300585; Tox21_500520; AC-472; BBL010484; CCG-38551; HB2775; LMPK12050218; s1342; STK801619; WHO 11073; ZINC18825330; AKOS001590147; Tox21_110161_1; CS-1534; DB01645; KS-5128; LP00520; SB17235; SDCCGSBI-0050503.P003; SMP1_000133; Genistein; 4',5,7-Trihydroxyisoflavone; NCGC00015479-01; NCGC00015479-02; NCGC00015479-04; NCGC00015479-05; NCGC00015479-06; NCGC00015479-07; NCGC00015479-08; NCGC00015479-10; NCGC00015479-11; NCGC00015479-12; NCGC00015479-13; NCGC00015479-14; NCGC00015479-15; NCGC00015479-16; NCGC00015479-17; NCGC00015479-18; NCGC00015479-19; NCGC00015479-20; NCGC00015479-38; NCGC00025005-01; NCGC00025005-02; NCGC00025005-03; NCGC00025005-04; NCGC00025005-05; NCGC00025005-06; NCGC00025005-07; NCGC00169711-01; NCGC00169711-02; NCGC00254275-01; NCGC00258979-01; NCGC00261205-01; 690224-00-1; HY-14596; NCI60_003369; SMR000112580; SY050124; EU-0100520; FT-0603395; FT-0668961; FT-0668962; G0272; SW203763-2; 46G720; C06563; D11680; EN300-210743; G-2535; Genistein, synthetic, >=98% (HPLC), powder; K00046; US8552057, 2; AB00052696_09; AB00052696_12; 5,7-dihydroxy-3-(4-hydroxyphenyl)-chromen-4-one; A826657; Q415957; GENISTEIN (CONSTITUENT OF RED CLOVER) [DSC]; Genistein, primary pharmaceutical reference standard; Q-100484; SR-01000075498-1; SR-01000075498-3; SR-01000075498-6; 3-(4-hydroxyphenyl)-5,7-bis(oxidanyl)chromen-4-one; BRD-K43797669-001-02-3; BRD-K43797669-001-03-1; BRD-K43797669-001-10-6; Genistein, from Glycine max (soybean), ~98% (HPLC); SR-01000075498-10; 5,7-dihydroxy-3-(4-hydroxyphenyl)-1-benzopyran-4-one; F0001-2388; GENISTEIN (CONSTITUENT OF SOY ISOFLAVONES) [DSC]; 4H-1-Benzopyran-4-one,7-dihydroxy-3-(4-hydroxyphenyl)-; 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4 H-1-benzopyran-4-one; Genistein, United States Pharmacopeia (USP) Reference Standard; Genistein, Pharmaceutical Secondary Standard; Certified Reference Material; 5,7-DIHYDROXY-3-(4-HYDROXYPHENYL)-4H-1-BENZOPYRAN-4-ONE; 4',5,7-TRIHYDROXYISOFLAVONE; PRUNETOL; GENISTEOL
|