NPs Basic Information
|
Name |
Vitexin
|
| Molecular Formula | C21H20O10 | |
| IUPAC Name* |
5,7-dihydroxy-2-(4-hydroxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one
|
|
| SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(O2)C(=C(C=C3O)O)[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)O
|
|
| InChI |
InChI=1S/C21H20O10/c22-7-14-17(27)18(28)19(29)21(31-14)16-11(25)5-10(24)15-12(26)6-13(30-20(15)16)8-1-3-9(23)4-2-8/h1-6,14,17-19,21-25,27-29H,7H2/t14-,17-,18+,19-,21+/m1/s1
|
|
| InChIKey |
SGEWCQFRYRRZDC-VPRICQMDSA-N
|
|
| Synonyms |
Vitexin; 3681-93-4; Apigenin 8-C-glucoside; Vitxein; 8-beta-D-Glucopyranosyl-apigenin; 9VP70K75OK; 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one; CHEBI:16954; MFCD00017456; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-8-beta-D-glucopyranosyl-2-(4-hydroxyphenyl)-; Flavone, 8-D-glucosyl-4',5,7-trihydroxy-; (1S)-1,5-anhydro-1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]-D-glucitol; 4H-1-Benzopyran-4-one, 8-.beta.-D-glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)-; 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-4H-chromen-4-one; 8-beta-D-Glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; EINECS 222-963-8; UNII-9VP70K75OK; Vitexin,(S); ORIENTOSIDE; VITEXINA; VITEXINE; VITEXIN [INCI]; VITEXIN [USP-RS]; VITEXIN [WHO-DD]; Vitexin, analytical standard; SCHEMBL25277; CHEMBL487417; DTXSID90190287; Apigenin 8-C-.beta.-D-glucoside; ACT02625; HY-N0013; ZINC4245684; BDBM50362886; HB4123; s9192; AKOS025311479; AC-6086; CCG-208516; 4H-1-Benzopyran-4-one, 8-beta-D-glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)-; NCGC00163642-01; Vitexin 100 microg/mL in Acetone:Water; 5,7-dihydroxy-2-(4-hydroxyphenyl)-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]chromen-4-one; AS-55909; (hydroxymethyl)tetrahydro-2H-pyran-2-yl)-; CS-0007090; APIGENIN-8-C-.BETA.-D-GLUCOPYRANOSIDE; C01460; 681V934; Q259075; Vitexin, primary pharmaceutical reference standard; Q-100437; 8-((2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-; 5,7,4'-Trihydroxyflavone 8-C-.beta.-D-glucopyranoside; Vitexin, United States Pharmacopeia (USP) Reference Standard; FLAVONE, 8-.BETA.-D-GLUCOPYRANOSYL-4',5,7-TRIHYDROXY-; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-8-.beta.-D-glucopyranosyl-2-(4-hydroxyphenyl)-
|
|
| CAS | 3681-93-4 | |
| PubChem CID | 5280441 | |
| ChEMBL ID | CHEMBL487417 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 432.4 | ALogp: | 0.2 |
| HBD: | 7 | HBA: | 10 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 177.0 | Aromatic Rings: | 4 |
| Heavy Atoms: | 31 | QED Weighted: | 0.308 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -6.222 | MDCK Permeability: | 0.00000547 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.991 |
| Human Intestinal Absorption (HIA): | 0.841 | 20% Bioavailability (F20%): | 0.934 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.017 | Plasma Protein Binding (PPB): | 89.60% |
| Volume Distribution (VD): | 0.903 | Fu: | 11.41% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.211 | CYP1A2-substrate: | 0.039 |
| CYP2C19-inhibitor: | 0.023 | CYP2C19-substrate: | 0.053 |
| CYP2C9-inhibitor: | 0.067 | CYP2C9-substrate: | 0.526 |
| CYP2D6-inhibitor: | 0.094 | CYP2D6-substrate: | 0.167 |
| CYP3A4-inhibitor: | 0.065 | CYP3A4-substrate: | 0.02 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.091 | Half-life (T1/2): | 0.765 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.11 | Human Hepatotoxicity (H-HT): | 0.175 |
| Drug-inuced Liver Injury (DILI): | 0.978 | AMES Toxicity: | 0.797 |
| Rat Oral Acute Toxicity: | 0.076 | Maximum Recommended Daily Dose: | 0.008 |
| Skin Sensitization: | 0.67 | Carcinogencity: | 0.072 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.134 |
| Respiratory Toxicity: | 0.085 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
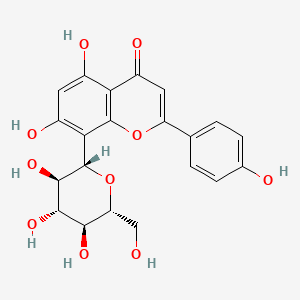
| ENC001575 | 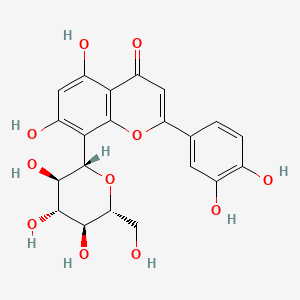 |
0.792 | D04AIT |  |
0.416 | ||
| ENC004475 | 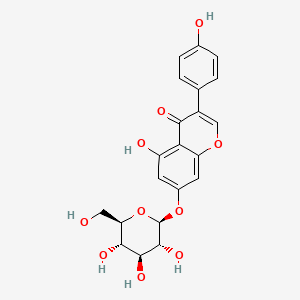 |
0.569 | D08DFX |  |
0.398 | ||
| ENC001572 | 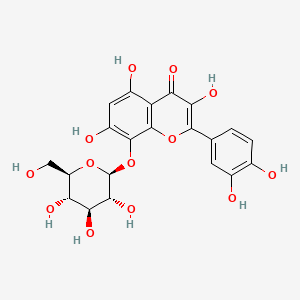 |
0.513 | D0TC7C | 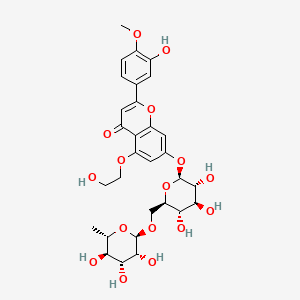 |
0.377 | ||
| ENC004734 | 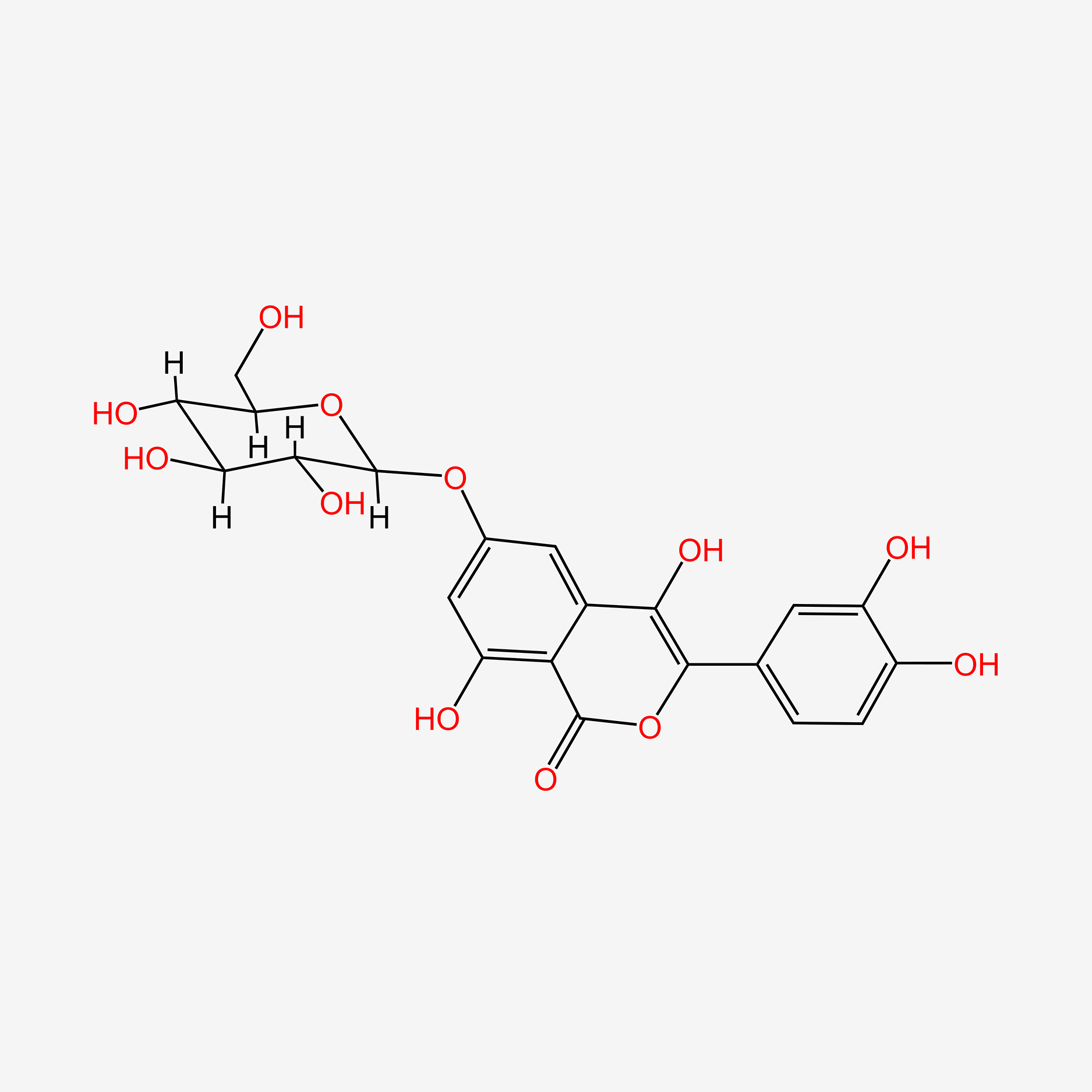 |
0.509 | D01TNW | 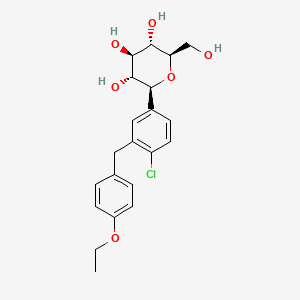 |
0.370 | ||
| ENC002201 |  |
0.508 | D06ALD |  |
0.352 | ||
| ENC001533 |  |
0.484 | D0AZ8C |  |
0.346 | ||
| ENC004476 |  |
0.482 | D0K8KX |  |
0.343 | ||
| ENC001573 |  |
0.460 | D06BQU |  |
0.321 | ||
| ENC001534 |  |
0.416 | D0I9HF |  |
0.310 | ||
| ENC001548 | 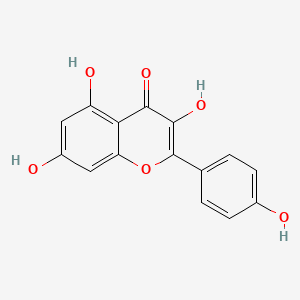 |
0.416 | D06GCK |  |
0.294 | ||