NPs Basic Information
|
Name |
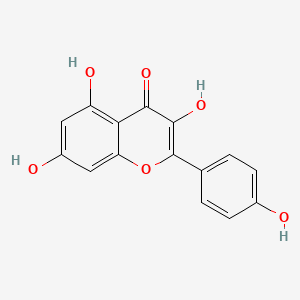
Kaempferol
|
| Molecular Formula | C15H10O6 | |
| IUPAC Name* |
3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
|
| SMILES |
C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O
|
|
| InChI |
InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
|
|
| InChIKey |
IYRMWMYZSQPJKC-UHFFFAOYSA-N
|
|
| Synonyms |
kaempferol; 520-18-3; Robigenin; Kaempherol; Kempferol; Populnetin; Rhamnolutein; Trifolitin; Swartziol; 3,4',5,7-Tetrahydroxyflavone; Pelargidenolon; Rhamnolutin; Indigo Yellow; Kampherol; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; Campherol; Kampferol; Nimbecetin; Kaemferol; 5,7,4'-Trihydroxyflavonol; Pelargidenolon 1497; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one; C.I. 75640; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-; CCRIS 41; Flavone, 3,4',5,7-tetrahydroxy-; Pelargidenon; Kampcetin; Kempferol;Robigenin; NSC 407289; NSC 656277; MFCD00016938; CHEMBL150; NSC-407289; NSC-656277; CHEBI:28499; 731P2LE49E; NSC656277; CAS-520-18-3; DSSTox_CID_768; DSSTox_RID_75781; DSSTox_GSID_20768; SMR000112585; EINECS 208-287-6; BRN 0304401; UNII-731P2LE49E; AI3-36096; 3,5,7,4'-Tetrahydroxyflavone; HSDB 7703; 4det; Kaempferol,(S); KAEMPFEROL [MI]; 5,4'-Trihydroxyflavonol; Prestwick0_001098; Prestwick1_001098; Prestwick2_001098; Prestwick3_001098; 3'-DEOXYQUERCETIN; KAEMPFEROL [HSDB]; KAEMPFEROL [IARC]; KAEMPFEROL [INCI]; 3,5,7-Tetrahydroxyflavone; 4',5,7-trihydroxyflavonol; KAEMPFEROL [USP-RS]; BIDD:PXR0073; Oprea1_650954; SCHEMBL18817; BSPBio_001176; 5-18-05-00251 (Beilstein Handbook Reference); MLS000697730; MLS001055391; MLS001074884; MLS006010737; BIDD:ER0134; SPBio_003058; Kaempferol, analytical standard; BDBM7462; BPBio1_001294; MEGxp0_001283; DTXSID7020768; Flavone,4',5,7-tetrahydroxy-; ACon1_001867; cid_5280863; GTPL11052; CHEBI: 28499; HMS1571K18; HMS2098K18; HMS2267I09; HMS3414C03; HMS3656M03; HMS3678C03; HMS3884B13; Kaempferol, >=97.0% (HPLC); TNP00039; ZINC3869768; Tox21_201165; Tox21_303363; AC-544; HSCI1_000027; LMPK12110003; NSC407289; s2314; AKOS015895240; Kaempferol, >=90% (HPLC), powder; CCG-202823; CS-1273; DB01852; GS-3570; NCGC00016480-01; NCGC00016480-02; NCGC00016480-03; NCGC00016480-04; NCGC00016480-05; NCGC00016480-06; NCGC00016480-07; NCGC00016480-08; NCGC00016480-09; NCGC00091036-01; NCGC00091036-02; NCGC00164322-01; NCGC00179275-01; NCGC00179275-02; NCGC00257464-01; NCGC00258717-01; BP-25390; CI 75640; HY-14590; Kaempferol 100 microg/mL in Acetonitrile; SY023424; AB00514046; FT-0614420; K0018; SW197199-2; 3,4',5,7-tetrahydroxy-Flavone (7CI,8CI); C05903; EN300-205764; H10428; S00111; Flavone, 3,4',5,7-tetrahydroxy- (7CI,8CI); KAEMPFEROL (CONSTITUENT OF GINKGO) [DSC]; 520K183; A828886; Q393336; SR-01000765646; Kaempferol, primary pharmaceutical reference standard; Q-100584; SR-01000765646-3; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-chromen-4-one; BRD-K12807006-001-05-2; BRD-K12807006-001-10-2; Z57183373; 2-(4-hydroxyphenyl)-3,5,7-tris(oxidanyl)chromen-4-one; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one??; A91A6666-86C8-4B33-B3EF-F74CD3CD7F47; 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-1-benzopyran-4-one; 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one #; 4H-1-Benzopyran-4-one,5,7-trihydroxy-2-(4-hydroxyphenyl)-; Kaempferol, United States Pharmacopeia (USP) Reference Standard; 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)- (9CI); 3,4',5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
|
|
| CAS | 520-18-3 | |
| PubChem CID | 5280863 | |
| ChEMBL ID | CHEMBL150 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 286.24 | ALogp: | 1.9 |
| HBD: | 4 | HBA: | 6 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 107.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 21 | QED Weighted: | 0.547 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.974 | MDCK Permeability: | 0.00000907 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.011 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.856 |
| 30% Bioavailability (F30%): | 0.993 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.009 | Plasma Protein Binding (PPB): | 97.86% |
| Volume Distribution (VD): | 0.522 | Fu: | 4.41% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.972 | CYP1A2-substrate: | 0.11 |
| CYP2C19-inhibitor: | 0.181 | CYP2C19-substrate: | 0.046 |
| CYP2C9-inhibitor: | 0.653 | CYP2C9-substrate: | 0.867 |
| CYP2D6-inhibitor: | 0.722 | CYP2D6-substrate: | 0.283 |
| CYP3A4-inhibitor: | 0.697 | CYP3A4-substrate: | 0.08 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.868 | Half-life (T1/2): | 0.905 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.07 | Human Hepatotoxicity (H-HT): | 0.098 |
| Drug-inuced Liver Injury (DILI): | 0.979 | AMES Toxicity: | 0.672 |
| Rat Oral Acute Toxicity: | 0.156 | Maximum Recommended Daily Dose: | 0.109 |
| Skin Sensitization: | 0.856 | Carcinogencity: | 0.097 |
| Eye Corrosion: | 0.009 | Eye Irritation: | 0.929 |
| Respiratory Toxicity: | 0.09 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001529 | 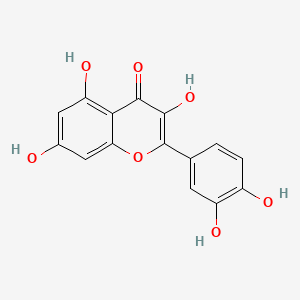 |
0.710 | D0K8KX |  |
0.710 | ||
| ENC001533 | 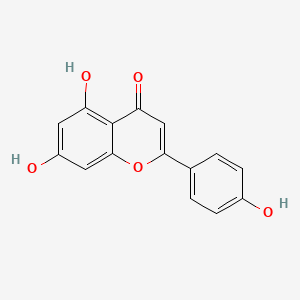 |
0.676 | D04AIT |  |
0.568 | ||
| ENC001550 | 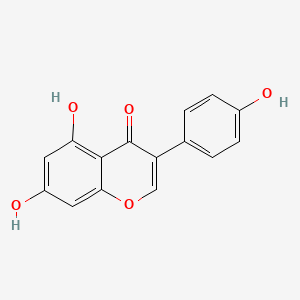 |
0.676 | D06GCK |  |
0.351 | ||
| ENC001534 | 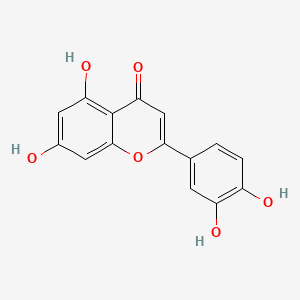 |
0.568 | D07MGA |  |
0.337 | ||
| ENC001573 | 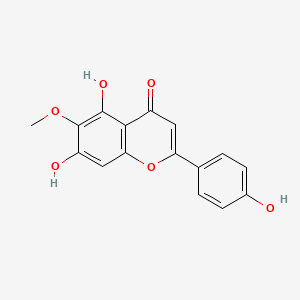 |
0.566 | D04XEG | 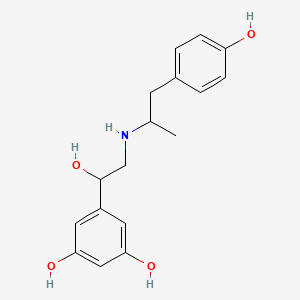 |
0.337 | ||
| ENC001771 | 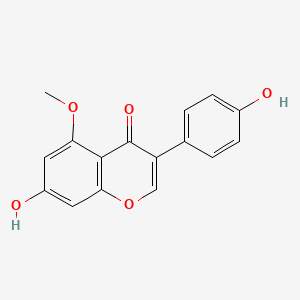 |
0.519 | D0R6BI | 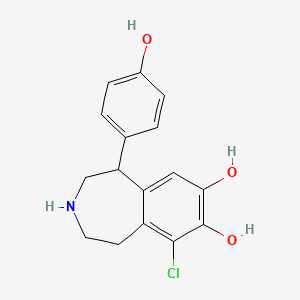 |
0.330 | ||
| ENC002018 | 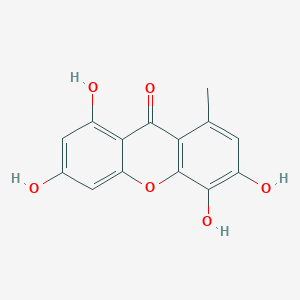 |
0.514 | D06TJJ | 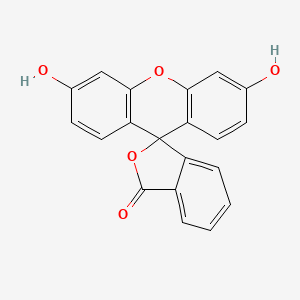 |
0.327 | ||
| ENC002024 | 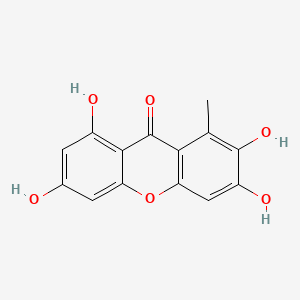 |
0.514 | D03UOT |  |
0.311 | ||
| ENC001574 | 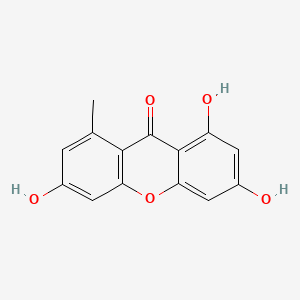 |
0.507 | D08LFZ | 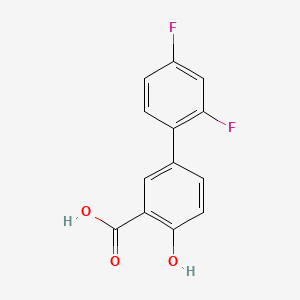 |
0.305 | ||
| ENC002757 | 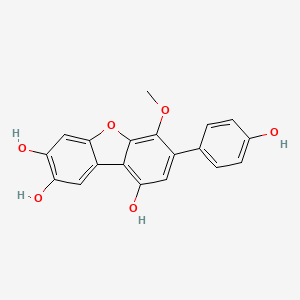 |
0.488 | D0U3YB | 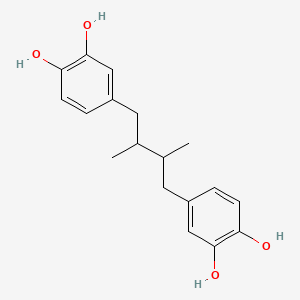 |
0.297 | ||