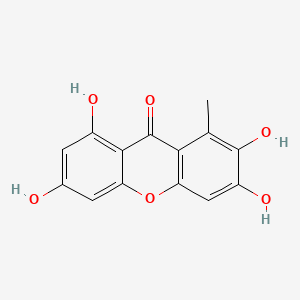
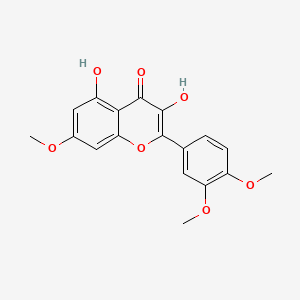
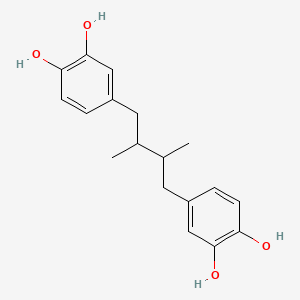
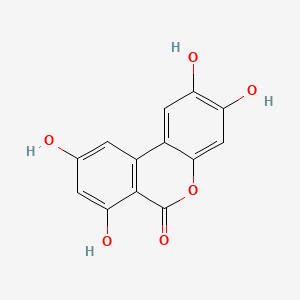
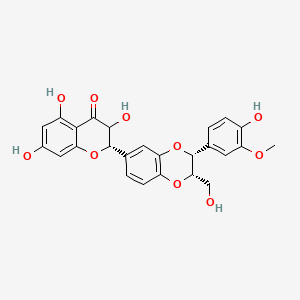
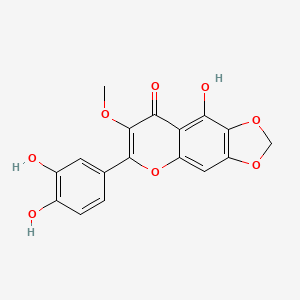
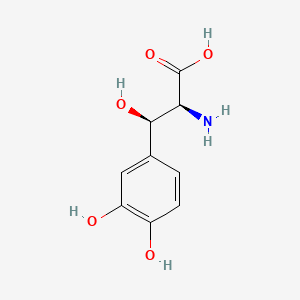
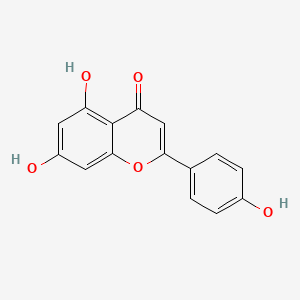
| Synonyms |
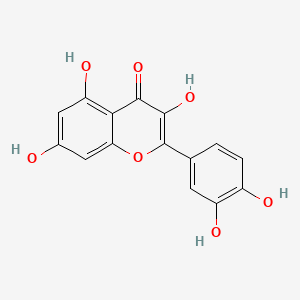
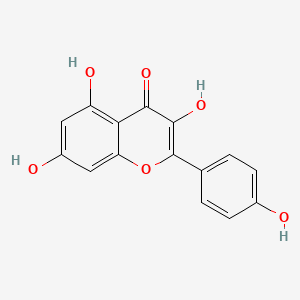
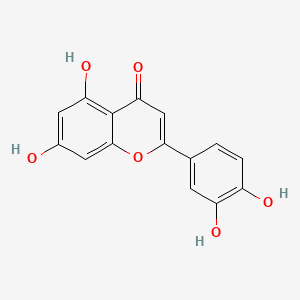
quercetin; 117-39-5; Meletin; Sophoretin; Quercetine; Xanthaurine; Quercetol; Quertine; 3,3',4',5,7-Pentahydroxyflavone; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one; Quercitin; Cyanidelonon 1522; 3,5,7,3',4'-Pentahydroxyflavone; Flavin meletin; Quertin; T-Gelb bzw. grun 1; C.I. Natural Yellow 10; Quercetin content; Kvercetin; C.I. 75670; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one; C.I. Natural red 1; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one; Corvitin; Cyanidenolon 1522; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-; 3',4',5,7-Tetrahydroxyflavan-3-ol; Flavone, 3,3',4',5,7-pentahydroxy-; C.I. Natural yellow 10 & 13; NSC 9219; Lipoflavon; CCRIS 1639; Korvitin; HSDB 3529; NCI-C60106; 3',4',5,7-tetrahydroxyflavon-3-ol; 3'-hydroxykaempferol; 3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-on; CHEBI:16243; AI3-26018; NSC9219; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one; NSC-9219; CHEMBL50; MFCD00006828; 9IKM0I5T1E; 2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one; 74893-81-5; Ci-75670; NSC-57655; Kvercetin [Czech]; LDN-0052529; Natural Yellow 10; Flavone, 3,4',5,5',7-pentahydroxy-; 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one; DSSTox_CID_1218; CI Natural Yellow 10; 7255-55-2; DSSTox_RID_76017; DSSTox_GSID_21218; QUE; BRD9794; BRD-9794; CAS-117-39-5; NSC57655; NSC58588; SR-01000076098; EINECS 204-187-1; UNII-9IKM0I5T1E; MixCom3_000183; BRN 0317313; CI 75670; Ritacetin; Quer; 4dfu; 4mra; Quercetin2H2O; Meletin;Sophoretin; KUC104418N; KUC107684N; 3,3',4,5,7-Pentahydroxyflavone; Quercetin-[d3]; LIM-5662; LNS-5662; TNP00070; TNP00089; KSC-23-76; Quercetin_sathishkumar; KSC-10-126; Quercetin (Sophoretin); Quercetin - Sophoretin; Spectrum_000124; Tocris-1125; 3cf8; QUERCETIN [DSC]; QUERCETIN [MI]; BiomolKI_000062; QUERCETIN [HSDB]; QUERCETIN [IARC]; QUERCETIN [INCI]; Maybridge1_008992; Prestwick0_000507; Prestwick1_000507; Prestwick2_000507; Prestwick3_000507; Spectrum2_000059; Spectrum3_000642; Spectrum4_000807; Spectrum5_001389; Lopac-Q-0125; QUERCETIN [VANDF]; P0042; C.I. natural yellow 13; BiomolKI2_000068; Enicostemma Littorale Blume; UPCMLD-DP081; Q 0125; QUERCETIN [USP-RS]; QUERCETIN [WHO-DD]; NCIOpen2_007628; NCIOpen2_007882; BIDD:PXR0007; Lopac0_000999; SCHEMBL19723; BSPBio_000433; BSPBio_001068; BSPBio_002243; KBioGR_000408; KBioGR_001293; KBioSS_000408; KBioSS_000584; 5-18-05-00494 (Beilstein Handbook Reference); MLS006011766; BIDD:ER0315; DivK1c_000485; SCHEMBL219729; SPECTRUM1500672; CU-01000012502-3; SPBio_000217; SPBio_002354; BDBM7460; BPBio1_000477; GTPL5346; MEGxp0_000381; SGCUT00001; 3,4',5,7-Pentahydroxyflavone; DTXSID4021218; NIOSH/LK8760000; UPCMLD-DP081:001; ACon1_000560; HMS501I07; KBio1_000485; KBio2_000408; KBio2_000584; KBio2_002976; KBio2_003152; KBio2_005544; KBio2_005720; KBio3_000775; KBio3_000776; KBio3_001463; 3,7,3',4'-Pentahydroxyflavone; NINDS_000485; Quercetin (constituent of ginkgo); 3',5,7-Tetrahydroxyflavan-3-ol; Bio1_000369; Bio1_000858; Bio1_001347; Bio2_000374; Bio2_000854; HMS1362F09; HMS1792F09; HMS1923O19; HMS1990F09; HMS3263G19; HMS3267M12; HMS3414J21; HMS3649D04; HMS3656C15; HMS3678J19; to_000078; ZINC3869685; 3,5,7,3',4'-Pentahydroxyflavon; Tox21_202308; Tox21_300285; Tox21_500999; BBL005513; CCG-40054; Flavone,3',4',5,7-pentahydroxy-; HB0542; LMPK12110004; NSC 57655; NSC324608; NSC756660; s2391; STK365650; Quercetin, >=95% (HPLC), solid; 3,4',5,5',7-pentahydroxy-Flavone; AKOS000511724; Quercetin 1000 microg/mL in Acetone; CS-3981; DB04216; DS-3416; LP00999; NSC-756660; SDCCGSBI-0050972.P003; IDI1_000485; IDI1_002129; LDN 0052529; SMP1_000252; NCGC00015870-01; NCGC00015870-02; NCGC00015870-03; NCGC00015870-04; NCGC00015870-05; NCGC00015870-06; NCGC00015870-07; NCGC00015870-08; NCGC00015870-09; NCGC00015870-10; NCGC00015870-11; NCGC00015870-12; NCGC00015870-13; NCGC00015870-14; NCGC00015870-15; NCGC00015870-16; NCGC00015870-17; NCGC00015870-18; NCGC00015870-19; NCGC00015870-21; NCGC00015870-22; NCGC00015870-23; NCGC00015870-24; NCGC00015870-25; NCGC00015870-28; NCGC00015870-48; NCGC00015870-50; NCGC00025016-01; NCGC00025016-02; NCGC00025016-03; NCGC00025016-04; NCGC00025016-05; NCGC00025016-06; NCGC00025016-07; NCGC00025016-08; NCGC00168962-01; NCGC00168962-02; NCGC00168962-03; NCGC00168962-04; NCGC00254218-01; NCGC00259857-01; NCGC00261684-01; Quercetin 100 microg/mL in Acetonitrile; AC-19596; AC-29756; HY-18085; NCI60_042036; SMR000112559; SY057722; (+)-3,3',4',5,7-Pentahydroxyflavone; Quercetin, Sophoretin, Meletin, Quercetine; EU-0100999; FT-0603318; FT-0655108; LK87600000; Q0025; SW148203-4; Quercetin; 3,3',4',5,7-Pentahydroxyflavone; 17Q395; C00389; EN300-199773; K00029; S00057; QUERCETIN (CONSTITUENT OF GINKGO) [DSC]; WLN: T66 BO EVJ CR CQ DQ & DQ GQ IQ; Flavone, 3,3',4',5,7-pentahydroxy-, (+)-; Q409478; Q-200333; SR-01000076098-1; SR-01000076098-3; SR-01000076098-7; SR-01000076098-8; BRD-K97399794-001-02-1; BRD-K97399794-001-07-0; BRD-K97399794-001-09-6; BRD-K97399794-001-11-2; BRD-K97399794-335-03-1; SR-01000076098-11; Z57176222; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate; 49643640-FD4C-4B93-BD28-0D7C2021CC52; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one #; (+)-4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-; 2-(3,4-DIHYDROXYPHENYL)-3,5,7-TRIHYDROXY-4H-BENZOPYRAN-4-ONE; 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-,zirconium(2+)salt(1:1); 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, zirconium(2+) salt (1:1)
|