NPs Basic Information
|
Name |
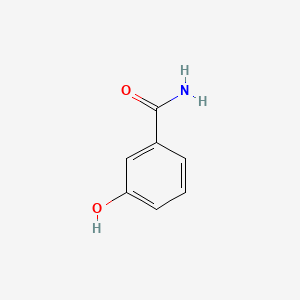
3-Hydroxybenzamide
|
| Molecular Formula | C7H7NO2 | |
| IUPAC Name* |
3-hydroxybenzamide
|
|
| SMILES |
C1=CC(=CC(=C1)O)C(=O)N
|
|
| InChI |
InChI=1S/C7H7NO2/c8-7(10)5-2-1-3-6(9)4-5/h1-4,9H,(H2,8,10)
|
|
| InChIKey |
NGMMGKYJUWYIIG-UHFFFAOYSA-N
|
|
| Synonyms |
3-hydroxybenzamide; 618-49-5; benzamide, 3-hydroxy-; 3-Hydroxy-benzamide; 3-hydroxybenzenecarboxamide; CHEMBL419424; NSC-379289; m-hydroxybenzamide; NSC379289; 3-OXIDANYLBENZAMIDE; 75X4NC5TNX; Oprea1_435073; SCHEMBL161861; 3-Hydroxybenzamide, AldrichCPR; DTXSID90321635; ZINC1590754; 3-HYDROXY-BENZOIC ACID,AMIDE; BDBM50068769; MFCD00017572; AKOS000207073; MB00281; SR-4267; NCGC00323509-01; SY004825; DB-008311; CS-0149666; FT-0600531; H1643; EN300-36686; C75901; AB01155218-03; A833450; AE-562/40219001; Z33546460
|
|
| CAS | 618-49-5 | |
| PubChem CID | 342403 | |
| ChEMBL ID | CHEMBL419424 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 137.14 | ALogp: | 0.4 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 63.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.622 | MDCK Permeability: | 0.00001470 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.99 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.016 |
| 30% Bioavailability (F30%): | 0.959 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.919 | Plasma Protein Binding (PPB): | 47.73% |
| Volume Distribution (VD): | 1.027 | Fu: | 67.34% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.461 | CYP1A2-substrate: | 0.31 |
| CYP2C19-inhibitor: | 0.057 | CYP2C19-substrate: | 0.059 |
| CYP2C9-inhibitor: | 0.025 | CYP2C9-substrate: | 0.537 |
| CYP2D6-inhibitor: | 0.08 | CYP2D6-substrate: | 0.389 |
| CYP3A4-inhibitor: | 0.026 | CYP3A4-substrate: | 0.154 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.151 | Half-life (T1/2): | 0.575 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.068 | Human Hepatotoxicity (H-HT): | 0.089 |
| Drug-inuced Liver Injury (DILI): | 0.402 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.056 | Maximum Recommended Daily Dose: | 0.011 |
| Skin Sensitization: | 0.207 | Carcinogencity: | 0.069 |
| Eye Corrosion: | 0.014 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.042 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000665 | 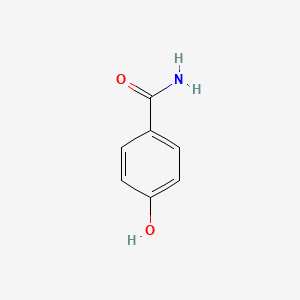 |
0.543 | D04EYC | 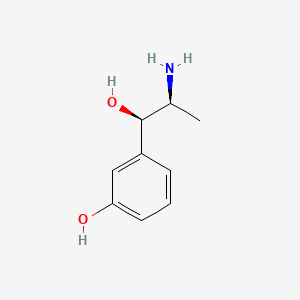 |
0.405 | ||
| ENC000076 | 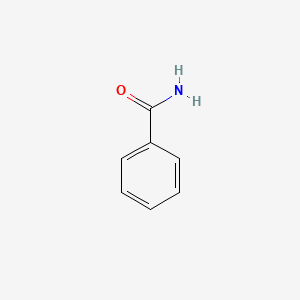 |
0.486 | D0S5LH | 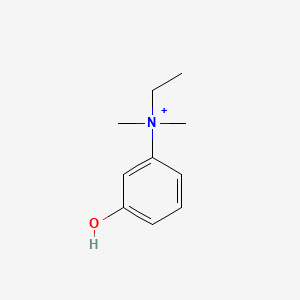 |
0.372 | ||
| ENC001055 | 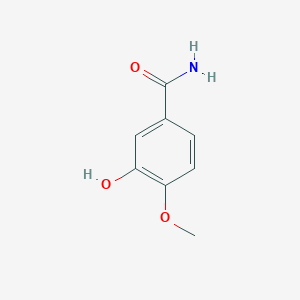 |
0.475 | D0O6IU | 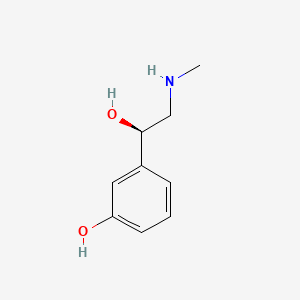 |
0.364 | ||
| ENC001056 | 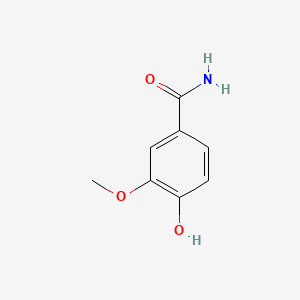 |
0.475 | D07HBX |  |
0.350 | ||
| ENC000108 | 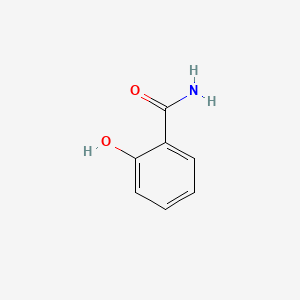 |
0.459 | D06NVJ | 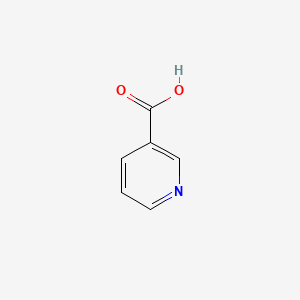 |
0.333 | ||
| ENC000048 | 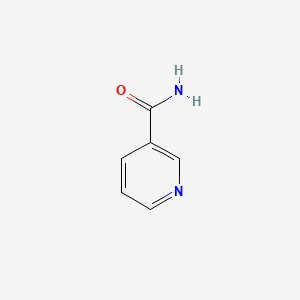 |
0.444 | D0X9RY |  |
0.333 | ||
| ENC000003 | 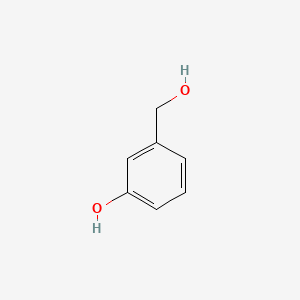 |
0.444 | D01WJL | 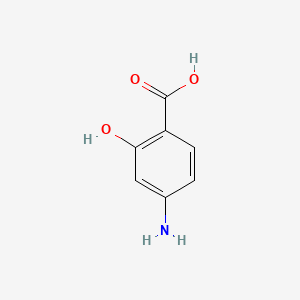 |
0.333 | ||
| ENC003374 | 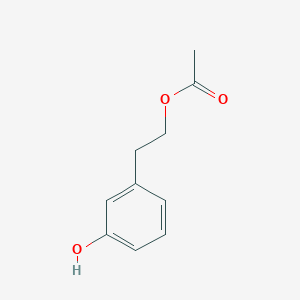 |
0.432 | D0C4YC | 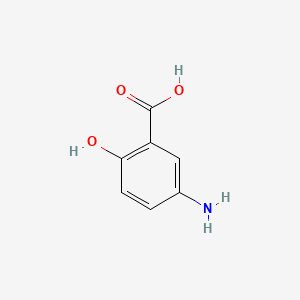 |
0.333 | ||
| ENC002426 | 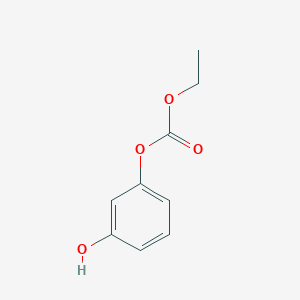 |
0.432 | D0U5QK |  |
0.326 | ||
| ENC000756 |  |
0.410 | D0S2BT |  |
0.326 | ||