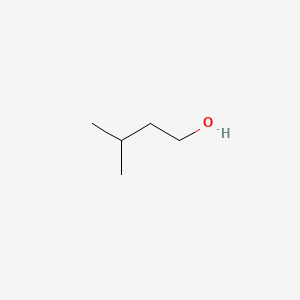
NPs Basic Information
|
Name |
Isoamyl alcohol
|
| Molecular Formula | C5H12O | |
| IUPAC Name* |
3-methylbutan-1-ol
|
|
| SMILES |
CC(C)CCO
|
|
| InChI |
InChI=1S/C5H12O/c1-5(2)3-4-6/h5-6H,3-4H2,1-2H3
|
|
| InChIKey |
PHTQWCKDNZKARW-UHFFFAOYSA-N
|
|
| Synonyms |
Isoamyl alcohol; 3-Methyl-1-butanol; Isopentyl alcohol; 3-Methylbutan-1-ol; 123-51-3; Isopentanol; 3-Methylbutanol; 1-Butanol, 3-methyl-; Isoamylol; Isobutylcarbinol; 2-Methyl-4-butanol; Iso-amylalkohol; Iso-amyl alcohol; Isobutyl carbinol; ISOAMYLALCOHOL; Alcool isoamylique; Fermentation amyl alcohol; Alcool amilico; Amylowy alkohol; Isoamyl alkohol; i-Amyl Alcohol; Primary isoamyl alcohol; 3-Metil-butanolo; isopentan-1-ol; Isoamyl alcohol (natural); MFCD00002934; FEMA No. 2057; isoamyl-alcohol; 3-Methyl-Butan-1-Ol; Isoamyl alcohol, primary; 3-methyl-Butanol; NSC 1029; Methyl-3-butan-1-ol; Butan-1-ol, 3-methyl; DEM9NIT1J4; Fuseloel; Huile de fusel; CHEBI:15837; 3-METHYL-BUTAN-(1)-OL; NSC-1029; NSC-7905; iso-pentanol; WLN: Q2Y1 & 1; FEMA Number 2057; Isoamyl alkohol [Czech]; Alcool amilico [Italian]; Amylowy alkohol [Polish]; Iso-amylalkohol [German]; 1-Hydroxy-3-Methylbutane; Alcool isoamylique [French]; 3-Metil-butanolo [Italian]; HSDB 605; 3-methylbutyl alcohol; EINECS 204-633-5; UNII-DEM9NIT1J4; iso-amylalcohol; isopentylalcohol; Isopentylalkohol; AI3-15288; CCRIS 8806; 3-methylbutanoI; 3-methyl butanol; 3-methyl 1-butanol; 3-methyl-1 butanol; 3-methylbutane-1-ol; Butanol, 3-methyl-; Isoamyl alcohol (primary and secondary); 6423-06-9; DSSTox_CID_5469; EC 204-633-5; DSSTox_RID_77799; DSSTox_GSID_25469; 3-Methyl-1-butanol, 98%; ISOAMYL ALCOHOL [FCC]; ISOAMYL ALCOHOL [FHFI]; ISOAMYL ALCOHOL [HSDB]; ISOAMYL ALCOHOL [INCI]; ISOPENTYL ALCOHOL [MI]; CHEMBL372396; QSPL 002; DTXSID3025469; Isoamyl alcohol, >=98%, FG; NSC1029; NSC7905; ZINC896830; Isoamyl alcohol (3-methyl butanol); 3-Methylbutanol, analytical standard; EINECS 229-179-5; Tox21_302359; LMFA05000108; STL282718; 3-Methyl-1-butanol A.C.S. Reagent; 3-Methyl-1-butanol, LR, >=98%; AKOS000118739; Magnesium bis(3-methylbutan-1-olate); NATURAL ISOAMYL ALCOHOL P & F; 3-METHYL-1-BUTANOL [USP-RS]; DB02296; 3-Methyl-1-butanol, p.a., 99.8%; Isoamyl alcohol, natural, >=98%, FG; 3-Methyl-1-butanol, analytical standard; NCGC00255329-01; 3-Methyl-1-butanol, anhydrous, >=99%; CAS-123-51-3; 3-Methyl-1-butanol, reagent grade, 98%; 3-Methyl-1-butanol, technical grade, 95%; ISOAMYL ALCOHOL ULTRA PURE GRADE 1L; FT-0616032; I0289; EN300-19333; 3-Methyl-1-butanol, ACS reagent, >=98.5%; 3-Methyl-1-butanol, biotech. grade, >=99%; 3-Methyl-1-butanol, ReagentPlus(R), >=99%; C07328; NATURAL ISOAMYL ALCOHOL - TECHNICAL GRADE; 3-Methyl-1-butanol, SAJ first grade, >=96.0%; Q223101; 3-Methyl-1-butanol, JIS special grade, >=98.0%; F0001-0367; Z104473558; 3-Methylbutanol, BioReagent, for molecular biology, >=98.5%; 3-Methylbutanol, puriss. p.a., ACS reagent, >=98.5% (GC); 3-Methylbutanol, BioUltra, for molecular biology, >=99.0% (GC); 3-Methyl-1-butanol, United States Pharmacopeia (USP) Reference Standard; 3-Methylbutanol, p.a., ACS reagent, reag. ISO, reag. Ph. Eur., 98.5%
|
|
| CAS | 123-51-3 | |
| PubChem CID | 31260 | |
| ChEMBL ID | CHEMBL372396 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 88.15 | ALogp: | 1.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 6 | QED Weighted: | 0.541 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.1 | MDCK Permeability: | 0.00004650 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.059 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.04 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.979 | Plasma Protein Binding (PPB): | 23.94% |
| Volume Distribution (VD): | 1.128 | Fu: | 68.34% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.424 | CYP1A2-substrate: | 0.441 |
| CYP2C19-inhibitor: | 0.037 | CYP2C19-substrate: | 0.804 |
| CYP2C9-inhibitor: | 0.031 | CYP2C9-substrate: | 0.65 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.148 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.212 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.289 | Half-life (T1/2): | 0.802 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.037 |
| Drug-inuced Liver Injury (DILI): | 0.039 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.064 | Maximum Recommended Daily Dose: | 0.02 |
| Skin Sensitization: | 0.238 | Carcinogencity: | 0.135 |
| Eye Corrosion: | 0.971 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.033 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000147 | 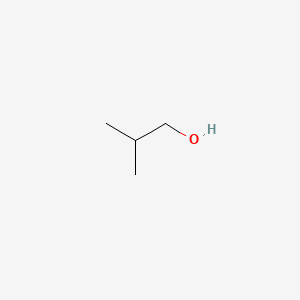 |
0.529 | D0C1QZ | 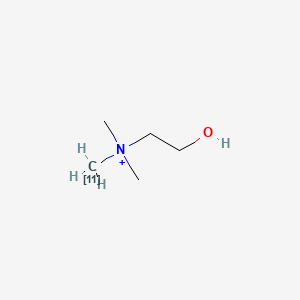 |
0.280 | ||
| ENC000396 | 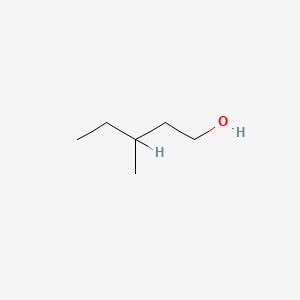 |
0.500 | D00WUF | 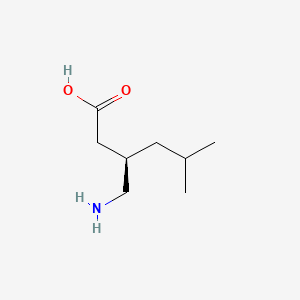 |
0.265 | ||
| ENC000445 | 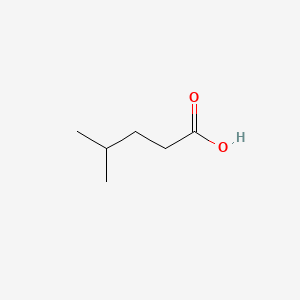 |
0.458 | D00AMQ | 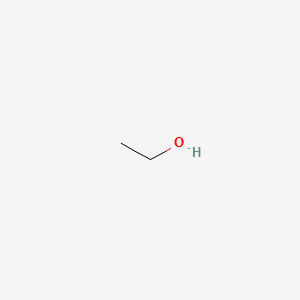 |
0.235 | ||
| ENC001474 | 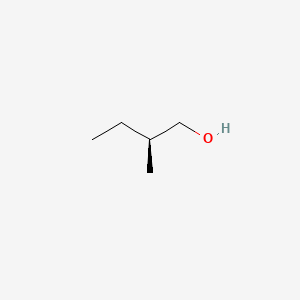 |
0.364 | D0R6BR | 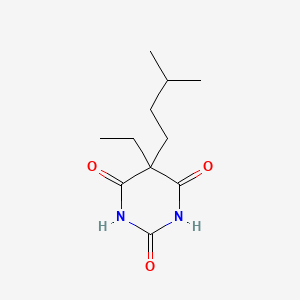 |
0.213 | ||
| ENC000307 | 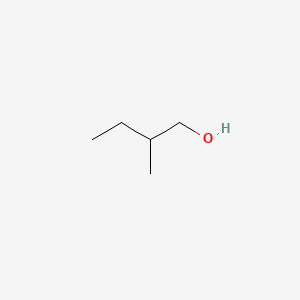 |
0.364 | D0Y3KG | 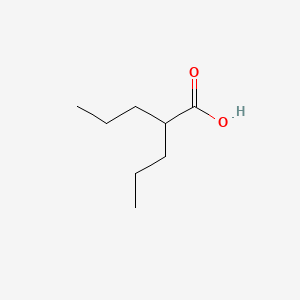 |
0.206 | ||
| ENC000603 | 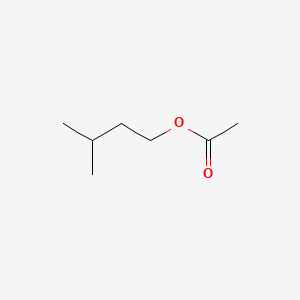 |
0.357 | D0M1PQ | 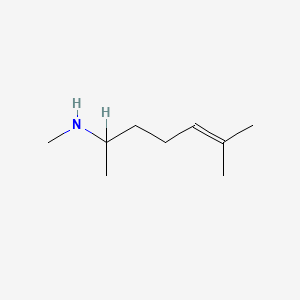 |
0.206 | ||
| ENC000311 | 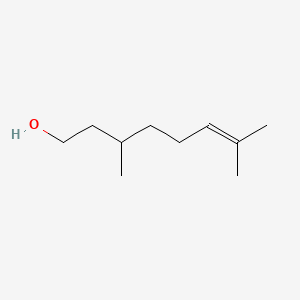 |
0.333 | D0X2IE | 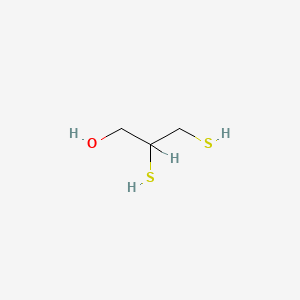 |
0.200 | ||
| ENC000351 | 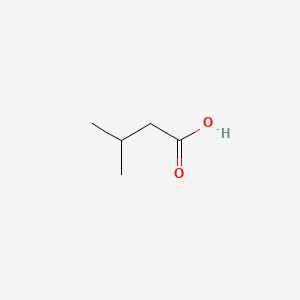 |
0.333 | D01OPV | 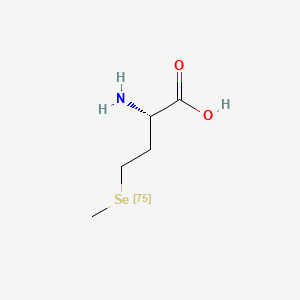 |
0.188 | ||
| ENC000503 | 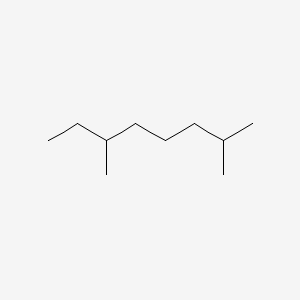 |
0.323 | D0EP8X | 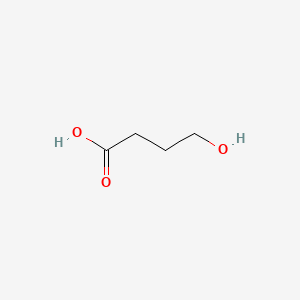 |
0.179 | ||
| ENC000227 | 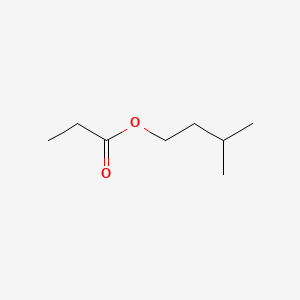 |
0.323 | D0R1QE | 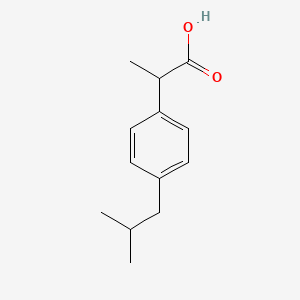 |
0.170 | ||