NPs Basic Information
|
Name |
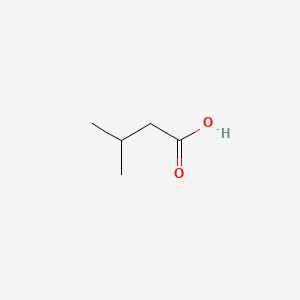
Isovaleric acid
|
| Molecular Formula | C5H10O2 | |
| IUPAC Name* |
3-methylbutanoic acid
|
|
| SMILES |
CC(C)CC(=O)O
|
|
| InChI |
InChI=1S/C5H10O2/c1-4(2)3-5(6)7/h4H,3H2,1-2H3,(H,6,7)
|
|
| InChIKey |
GWYFCOCPABKNJV-UHFFFAOYSA-N
|
|
| Synonyms |
ISOVALERIC ACID; 3-Methylbutanoic acid; 503-74-2; 3-Methylbutyric acid; Isopentanoic acid; Delphinic acid; Butanoic acid, 3-methyl-; Isopropylacetic acid; Isovalerianic acid; Isobutylformic acid; 3-Methylbutyrate; beta-Methylbutyric acid; Isovalerianic; Acetic acid, isopropyl-; Butyric acid, 3-methyl-; 3-methyl-butanoic acid; isovalerate; Kyselina isovalerova; 3-methyl butyric acid; 3-methyl-butyric acid; FEMA No. 3102; 3-methyl-n-butyric acid; Isovaleriansaeure; .beta.-Methylbutyric acid; MFCD00002726; NSC 62783; 3-Methylbuttersaeure; b-Methylbutyric acid; 3,4-Diisovaleryl adrenaline; CHEBI:28484; 1BR7X184L5; Butanoic acid, 3-methyl-, (R)-; isopropylacetate; NSC-62783; IVA; Isobutyl formic acid; Isovaleric acid (natural); FEMA Number: 3102; Kyselina isovalerova [Czech]; methyl butanoic acid; HSDB 629; EINECS 207-975-3; METHYLBUTANOIC ACID; BRN 1098522; UNII-1BR7X184L5; AI3-24132; b-Methylbutyrate; iso-valeric acid; 3-Methylbutanoicacid; DELPHINIC-ACID; iso-C4H9COOH; Isovaleric acid, 99%; DSSTox_CID_9182; bmse000373; EC 207-975-3; DSSTox_RID_78698; DSSTox_GSID_29182; SCHEMBL43436; ISOVALERIC ACID [MI]; 4-02-00-00895 (Beilstein Handbook Reference); ISOVALERIC ACID [FCC]; NATURAL ISOVALERIC ACID; Isopropyl Acetic Acid, natural; ISOVALERIC ACID [FHFI]; ISOVALERIC ACID [HSDB]; CHEMBL568737; WLN: QV1Y1&1; DTXSID5029182; ISOVALERIC ACID [MART.]; ISOVALERIC ACID [WHO-DD]; ZINC388188; AMY40214; BCP32116; NSC62783; STR08356; Isovaleric acid, analytical standard; Tox21_201604; BBL027399; LMFA01020181; s6287; STL146358; AKOS000119861; Isovaleric acid, >=99%, FCC, FG; CS-W013696; DB03750; HY-W012980; 3-Methylbutyric acid: isopropyl-Acetate; Isovaleric acid sodium salt (Salt/Mix); Isovaleric acid, natural, >=98%, FG; NCGC00249082-01; NCGC00259153-01; 35915-22-1; CAS-503-74-2; 3-Methylbutyric acid: isopropyl-Acetic acid; FT-0627533; M0182; EN300-19718; C08262; D78213; Q415536; J-522594; F2191-0067; Z104474910; 3-Methylbutanoic acid;3-Methylbutyric acid;Isopentanoic acid; 92634-50-9
|
|
| CAS | 503-74-2 | |
| PubChem CID | 10430 | |
| ChEMBL ID | CHEMBL568737 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 102.13 | ALogp: | 1.2 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.574 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.685 | MDCK Permeability: | 0.00009820 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.004 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.946 | Plasma Protein Binding (PPB): | 40.33% |
| Volume Distribution (VD): | 0.257 | Fu: | 64.67% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.03 | CYP1A2-substrate: | 0.095 |
| CYP2C19-inhibitor: | 0.021 | CYP2C19-substrate: | 0.472 |
| CYP2C9-inhibitor: | 0.01 | CYP2C9-substrate: | 0.961 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.145 |
| CYP3A4-inhibitor: | 0.007 | CYP3A4-substrate: | 0.078 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.96 | Half-life (T1/2): | 0.807 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.006 | Human Hepatotoxicity (H-HT): | 0.132 |
| Drug-inuced Liver Injury (DILI): | 0.286 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.32 | Maximum Recommended Daily Dose: | 0.016 |
| Skin Sensitization: | 0.292 | Carcinogencity: | 0.076 |
| Eye Corrosion: | 0.979 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.049 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000031 | 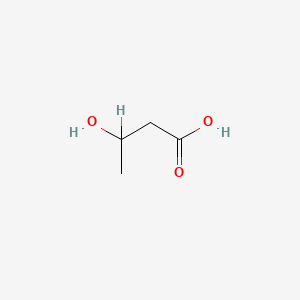 |
0.545 | D00WUF | 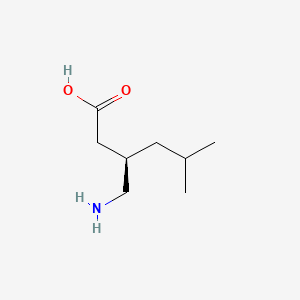 |
0.500 | ||
| ENC000376 | 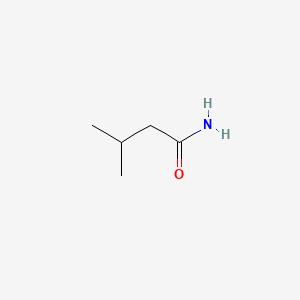 |
0.545 | D09PUL | 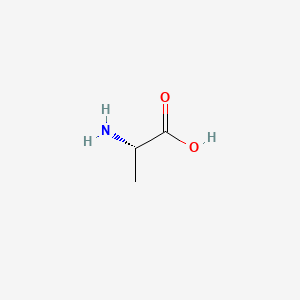 |
0.364 | ||
| ENC000237 | 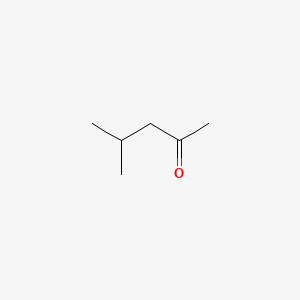 |
0.545 | D08QGD | 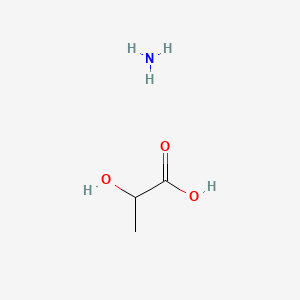 |
0.348 | ||
| ENC000445 | 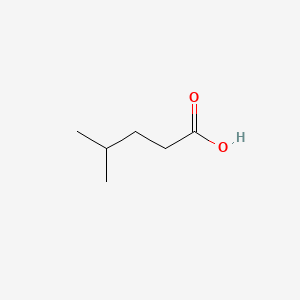 |
0.542 | D0M8AB | 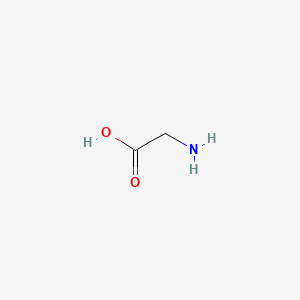 |
0.333 | ||
| ENC000149 | 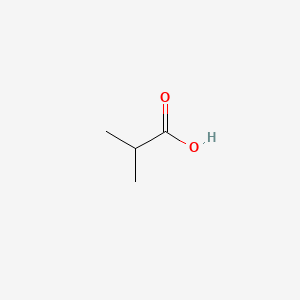 |
0.500 | D02KJX | 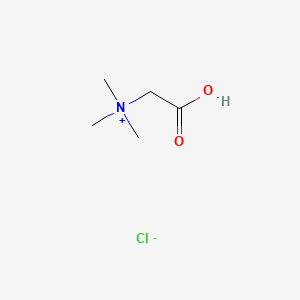 |
0.321 | ||
| ENC000685 | 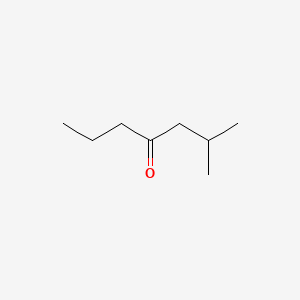 |
0.429 | D04CRL | 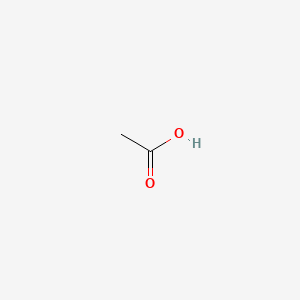 |
0.316 | ||
| ENC000241 | 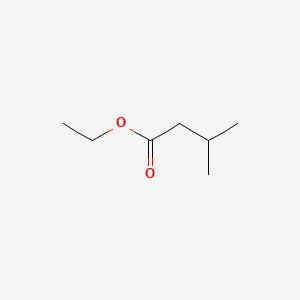 |
0.429 | D0A8CJ | 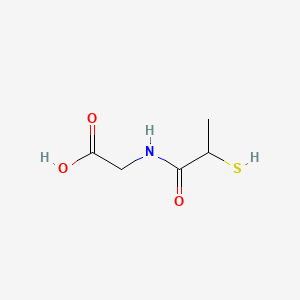 |
0.313 | ||
| ENC000289 | 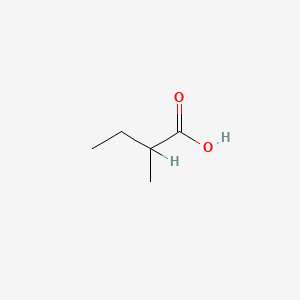 |
0.417 | D00ZOF | 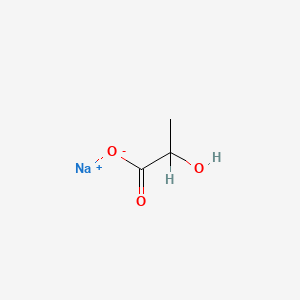 |
0.292 | ||
| ENC000147 | 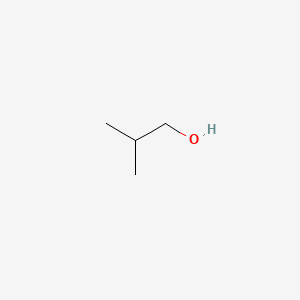 |
0.400 | D0ZK8H | 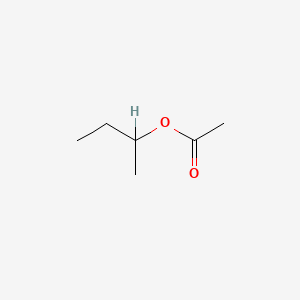 |
0.276 | ||
| ENC000058 | 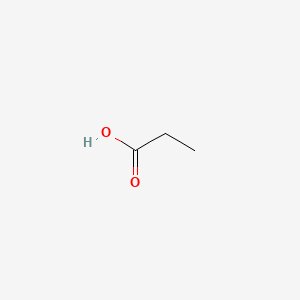 |
0.400 | D08HZC |  |
0.267 | ||