NPs Basic Information
|
Name |
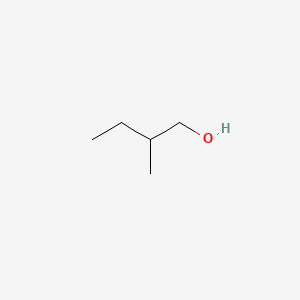
2-Methyl-1-butanol
|
| Molecular Formula | C5H12O | |
| IUPAC Name* |
2-methylbutan-1-ol
|
|
| SMILES |
CCC(C)CO
|
|
| InChI |
InChI=1S/C5H12O/c1-3-5(2)4-6/h5-6H,3-4H2,1-2H3
|
|
| InChIKey |
QPRQEDXDYOZYLA-UHFFFAOYSA-N
|
|
| Synonyms |
2-METHYL-1-BUTANOL; 2-Methylbutan-1-ol; 137-32-6; Active amyl alcohol; sec-Butylcarbinol; 1-Butanol, 2-methyl-; 2-Methylbutanol; DL-2-Methyl-1-butanol; 2-Methyl-n-butanol; 2-Methylbutyl alcohol; Primary active amyl alcohol; 2-Methyl butanol-1; Active primary amyl alcohol; dl-sec-Butyl carbinol; (+/-)-2-Methyl-1-butanol; Methyl-2-butan-1-ol; NSC 8431; sec-Butyl carbinol; (1)-2-Methylbutan-1-ol; CH3CH2CH(CH3)CH2OH; 7VTJ239ASU; L-2-Methyl-1-butanol; CHEBI:48945; NSC-8431; DSSTox_CID_7069; DSSTox_RID_78299; DSSTox_GSID_27069; 2-methyl butanol; (-)-2-methylbutanol; CAS-137-32-6; HSDB 5626; 2-Methyl-Butan-1-Ol; EINECS 205-289-9; EINECS 252-163-4; 34713-94-5; UNII-7VTJ239ASU; BRN 1718810; AI3-24190; CCRIS 8805; D-2-METHYL-1-BUTANOL; 2-methyl-butanol; ( inverted exclamation markA)-2-Methyl-1-butanol; MFCD00004743; DL-sec-Butylcarbinol; (-)2-methylbutanol; 2-methyl 1-butanol; Butanol, 2-methyl-; DL-2-METHYL-1-BUTANOL, PRACT; 3-Methyl iso-butanol; (+)-2-methylbutanol; Nat.L-2-Methylbutanol; EC 205-289-9; (RS)-2-methyl-1-butanol; 2-Methyl-(S)-1-Butanol; 4-01-00-01666 (Beilstein Handbook Reference); 2-Methyl-(2S)-1-Butanol; CHEMBL451923; DTXSID5027069; FEMA NO. 3998; 2-Methyl-1-butanol, >=99%; NSC8431; 2-METHYL-1-BUTANOL [MI]; WLN: Q1Y2 & 1; 2-Methyl-(.+/-.)-1-Butanol; 2-METHYL-1-BUTANOL [HSDB]; Tox21_201558; Tox21_303200; LMFA05000104; STL185573; 2-Methyl-1-butanol, >=99%, FG; AKOS009159118; 2-Methyl-1-butanol, analytical standard; 2-METHYL-1-BUTANOL,(+/-)-; NCGC00249069-01; NCGC00256976-01; NCGC00259107-01; 2-Methyl-1-butanol, natural, 99%, FG; DB-003288; FT-0605210; FT-0612896; FT-0691797; M0175; (+/-)-2-METHYL-1-BUTANOL [FHFI]; EN300-126214; Q209425; (+/-)-2-Methyl-1-butanol, >=98.0% (GC); J-510045; F0001-0469
|
|
| CAS | 137-32-6 | |
| PubChem CID | 8723 | |
| ChEMBL ID | CHEMBL451923 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 88.15 | ALogp: | 1.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 6 | QED Weighted: | 0.541 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.177 | MDCK Permeability: | 0.00003280 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.034 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.007 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.993 | Plasma Protein Binding (PPB): | 31.04% |
| Volume Distribution (VD): | 1.005 | Fu: | 71.16% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.359 | CYP1A2-substrate: | 0.737 |
| CYP2C19-inhibitor: | 0.035 | CYP2C19-substrate: | 0.727 |
| CYP2C9-inhibitor: | 0.017 | CYP2C9-substrate: | 0.207 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.26 |
| CYP3A4-inhibitor: | 0.009 | CYP3A4-substrate: | 0.23 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.58 | Half-life (T1/2): | 0.803 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.018 | Human Hepatotoxicity (H-HT): | 0.028 |
| Drug-inuced Liver Injury (DILI): | 0.055 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.182 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.271 | Carcinogencity: | 0.189 |
| Eye Corrosion: | 0.903 | Eye Irritation: | 0.99 |
| Respiratory Toxicity: | 0.098 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001474 | 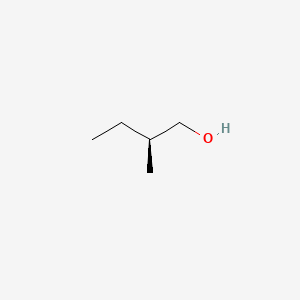 |
1.000 | D00AMQ | 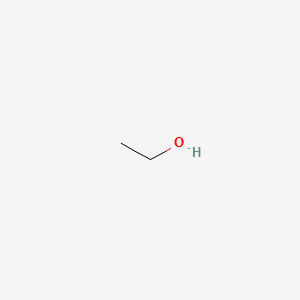 |
0.313 | ||
| ENC000396 | 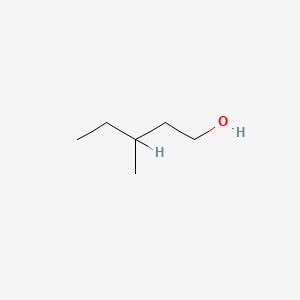 |
0.571 | D0X2IE | 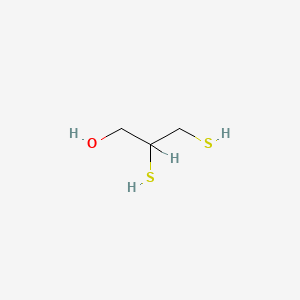 |
0.250 | ||
| ENC000182 |  |
0.500 | D0ZK8H | 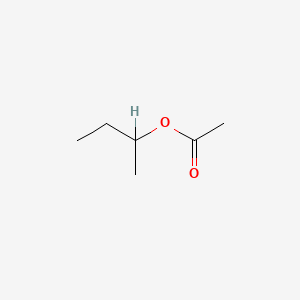 |
0.250 | ||
| ENC000147 | 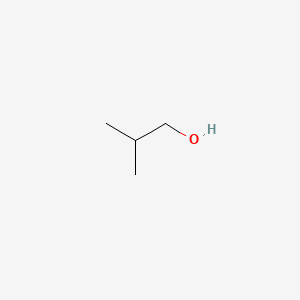 |
0.444 | D08QME | 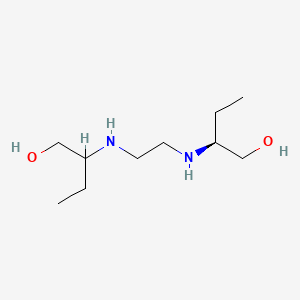 |
0.233 | ||
| ENC000225 | 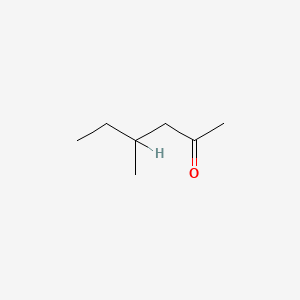 |
0.400 | D0C1QZ | 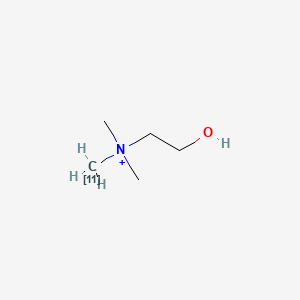 |
0.231 | ||
| ENC001246 | 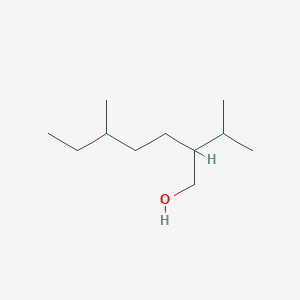 |
0.394 | D0Y3KG | 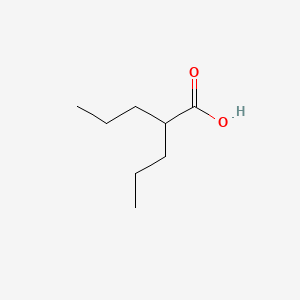 |
0.206 | ||
| ENC000057 |  |
0.368 | D00WUF |  |
0.194 | ||
| ENC001788 |  |
0.367 | D02UDJ |  |
0.185 | ||
| ENC000600 |  |
0.364 | D0K4MH |  |
0.184 | ||
| ENC001138 |  |
0.357 | D0A4JK |  |
0.174 | ||