NPs Basic Information
|
Name |
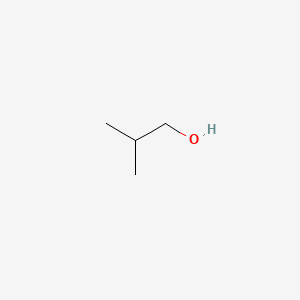
Isobutanol
|
| Molecular Formula | C4H10O | |
| IUPAC Name* |
2-methylpropan-1-ol
|
|
| SMILES |
CC(C)CO
|
|
| InChI |
InChI=1S/C4H10O/c1-4(2)3-5/h4-5H,3H2,1-2H3
|
|
| InChIKey |
ZXEKIIBDNHEJCQ-UHFFFAOYSA-N
|
|
| Synonyms |
2-Methyl-1-propanol; Isobutanol; ISOBUTYL ALCOHOL; 2-Methylpropan-1-ol; 78-83-1; 1-Propanol, 2-methyl-; 1-Hydroxymethylpropane; Isopropylcarbinol; Iso-butyl alcohol; 2-Methylpropyl alcohol; i-Butyl alcohol; Isobutylalkohol; 2-Methylpropanol; Alcool isobutylique; 2-Methyl propanol; Fermentation butyl alcohol; i-Butanol; RCRA waste number U140; 2-Methylpropanol-1; FEMA No. 2179; Isopropyl carbitol; NSC 5708; Methyl-2 propanol-1; iso-C4H9OH; MFCD00004740; 2-methyl-1-propanyl alcohol; 56F9Z98TEM; CHEBI:46645; NSC-5708; Isobutylalkohol [Czech]; iso-butanol; FEMA Number 2179; Isobutyl alcohol (natural); Isopropyl carbinol; Alcool isobutylique [French]; HSDB 49; CCRIS 2300; EINECS 201-148-0; UN1212; RCRA waste no. U140; BRN 1730878; isobutylalcohol; UNII-56F9Z98TEM; iso butanol; Butanol-iso; AI3-01777; 2-methylpropanoI; iBuOH; 2-methyl-propanol; iso-BuOH; i-BuOH; 2-methyl-l-propanol; 2-methyl-n-propanol; Isobutanol ACS grade; Propanol, 2-methyl-; 2-methyl-propan-1-ol; Isobutanol, HPLC Grade; DSSTox_CID_1759; EC 201-148-0; DSSTox_RID_76310; Isobutanol, Isobutyl alcohol; DSSTox_GSID_21759; Isobutyl Alcohol Reagent ACS; 4-01-00-01588 (Beilstein Handbook Reference); BIDD:ER0628; Isobutanol or isobutyl alcohol; ISOBUTYL ALCOHOL [II]; ISOBUTYL ALCOHOL [MI]; 2-Methyl-1-propanol, 99%; ISOBUTYL ALCOHOL [FCC]; NATURAL ISOBUTYL ALCOHOL; CHEMBL269630; ISOBUTYL ALCOHOL [FHFI]; ISOBUTYL ALCOHOL [HSDB]; DTXSID0021759; WLN: Q1Y1&1; ISOBUTYL ALCOHOL [MART.]; NSC5708; 2-Methyl-1-propanol, 99.5%; Isobutyl Alcohol (Fragrance Grade); 2-Methyl-1-propanol, AR, 99%; ACT03408; Isobutanol, Spectrophotometric Grade; ZINC1687155; Tox21_201214; ISOBUTYL ALCOHOL (ISOBUTANOL); LMFA05000100; STL185664; 2-methyl-1-propanol(isobutyl alcohol); 2-Methyl-1-propanol, LR, >=99%; AKOS000118740; Isobutyl alcohol, >=99%, FCC, FG; UN 1212; 2-METHYL-1-PROPANOL [USP-RS]; CAS-78-83-1; NCGC00091851-01; NCGC00091851-02; NCGC00258766-01; 2-Methyl-1-propanol, analytical standard; 68989-27-5; 2-Methyl-1-propanol, anhydrous, 99.5%; 2-Methyl-1-propanol, for HPLC, 99.5%; Isobutyl alcohol, ACS reagent, >=99.0%; FT-0627343; I0094; EN300-19336; Isobutyl alcohol 5000 microg/mL in Methanol; 2-Methyl-1-propanol 10 microg/mL in Methanol; Isobutyl alcohol, natural, >=99%, FCC, FG; 2-Methyl-1-propanol, ACS reagent, >=99.0%; Q151797; 2-Methyl-1-propanol, p.a., ACS reagent, 99.0%; 2-Methyl-1-propanol, SAJ first grade, >=99.0%; J-509912; 2-Methyl-1-propanol, JIS special grade, >=99.0%; F0001-2058; 2-Methyl-1-propanol, ACS spectrophotometric grade, >=99.0%; 2-Methyl-1-propanol, reag. ISO, 99%, UV HPLC spectroscopic; Isobutanol or isobutyl alcohol [UN1212] [Flammable liquid]; 2-Methyl-1-propanol, puriss. p.a., ACS reagent, >=99.5% (GC); 2-Methyl-1-propanol, BioUltra, for molecular biology, >=99.5% (GC); 2-Methyl-1-propanol, United States Pharmacopeia (USP) Reference Standard; 2-Methyl-1-propanol, puriss. p.a., ACS reagent, reag. Ph. Eur., >=99% (GC); 5OZ
|
|
| CAS | 78-83-1 | |
| PubChem CID | 6560 | |
| ChEMBL ID | CHEMBL269630 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 74.12 | ALogp: | 0.8 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 5 | QED Weighted: | 0.492 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.168 | MDCK Permeability: | 0.00016727 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.053 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.025 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.987 | Plasma Protein Binding (PPB): | 20.09% |
| Volume Distribution (VD): | 1.069 | Fu: | 82.96% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.397 | CYP1A2-substrate: | 0.377 |
| CYP2C19-inhibitor: | 0.032 | CYP2C19-substrate: | 0.816 |
| CYP2C9-inhibitor: | 0.02 | CYP2C9-substrate: | 0.362 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.312 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.235 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.625 | Half-life (T1/2): | 0.826 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.016 | Human Hepatotoxicity (H-HT): | 0.029 |
| Drug-inuced Liver Injury (DILI): | 0.126 | AMES Toxicity: | 0.014 |
| Rat Oral Acute Toxicity: | 0.213 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.233 | Carcinogencity: | 0.097 |
| Eye Corrosion: | 0.891 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.03 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000600 | 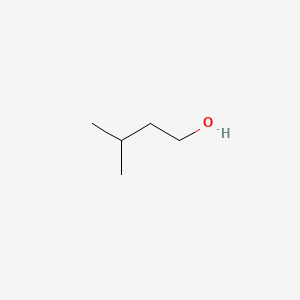 |
0.529 | D00AMQ | 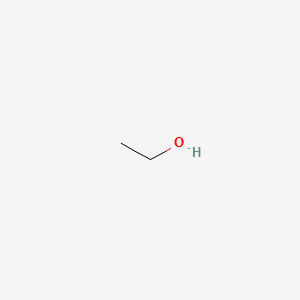 |
0.308 | ||
| ENC000057 | 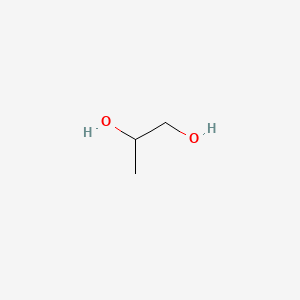 |
0.467 | D00WUF | 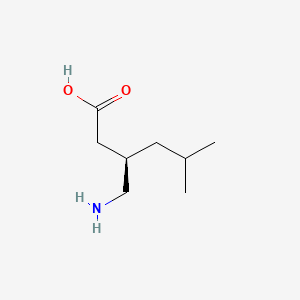 |
0.258 | ||
| ENC000307 | 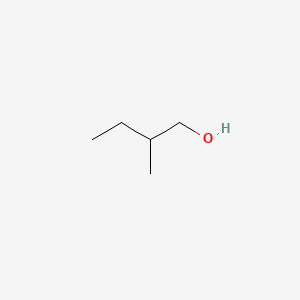 |
0.444 | D0X2IE | 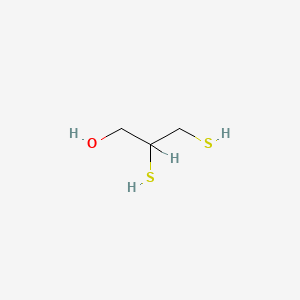 |
0.238 | ||
| ENC001474 | 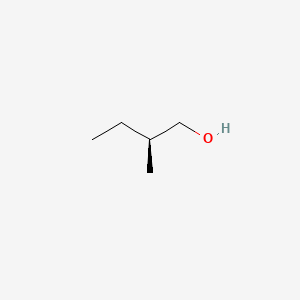 |
0.444 | D0C1QZ | 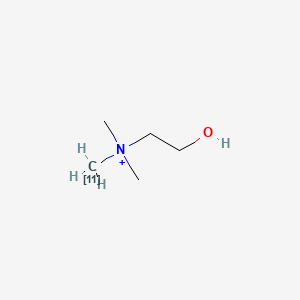 |
0.217 | ||
| ENC000351 | 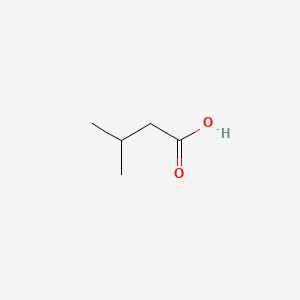 |
0.400 | D02UDJ | 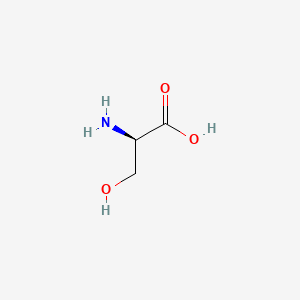 |
0.217 | ||
| ENC000445 | 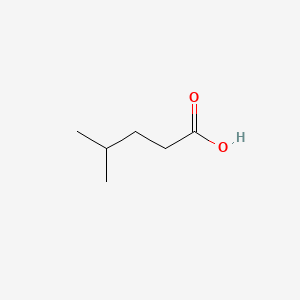 |
0.348 | D09PUL | 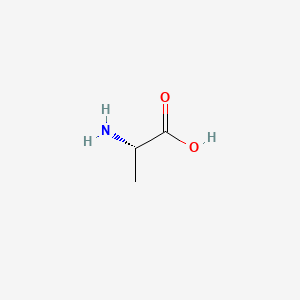 |
0.200 | ||
| ENC000149 | 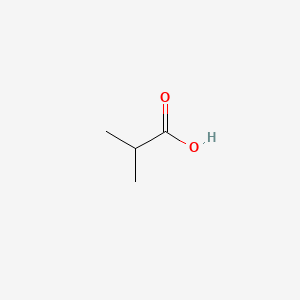 |
0.333 | D0ZK8H | 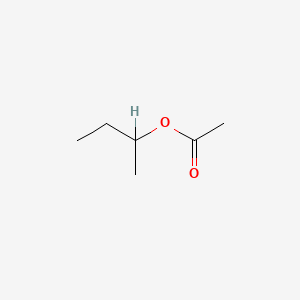 |
0.192 | ||
| ENC000376 | 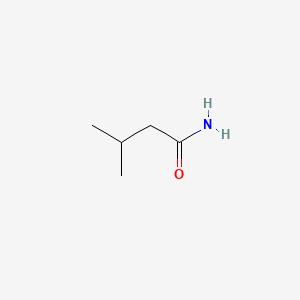 |
0.333 | D08QGD | 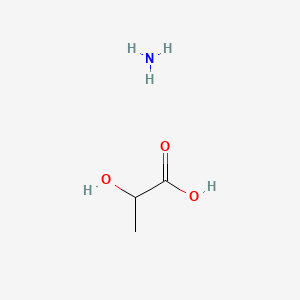 |
0.190 | ||
| ENC001011 | 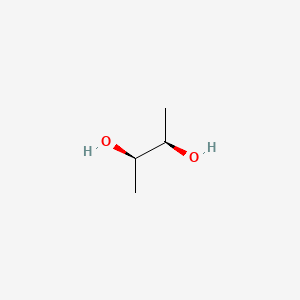 |
0.333 | D00ZOF | 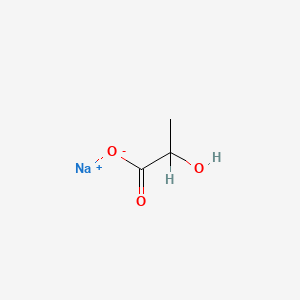 |
0.190 | ||
| ENC000016 | 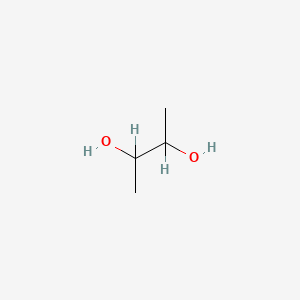 |
0.333 | D01JQJ | 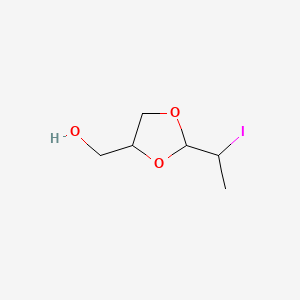 |
0.188 | ||