NPs Basic Information
|
Name |
Citronellol
|
| Molecular Formula | C10H20O | |
| IUPAC Name* |
3,7-dimethyloct-6-en-1-ol
|
|
| SMILES |
CC(CCC=C(C)C)CCO
|
|
| InChI |
InChI=1S/C10H20O/c1-9(2)5-4-6-10(3)7-8-11/h5,10-11H,4,6-8H2,1-3H3
|
|
| InChIKey |
QMVPMAAFGQKVCJ-UHFFFAOYSA-N
|
|
| Synonyms |
Citronellol; 106-22-9; 3,7-Dimethyloct-6-en-1-ol; beta-Citronellol; DL-Citronellol; Cephrol; 6-Octen-1-ol, 3,7-dimethyl-; 3,7-DIMETHYL-6-OCTEN-1-OL; Elenol; Rodinol; 2,3-Dihydrogeraniol; 2,6-Dimethyl-2-octen-8-ol; (+/-)-beta-Citronellol; Citronellol, dl-; .beta.-Citronellol; Dihydrogeraniol; 26489-01-0; (+/-)-3,7-dimethyloct-6-en-1-ol; CHEBI:50462; NSC 8779; 565OK72VNF; 3,7-dimethyl-oct-6-en-1-ol; NSC8779; NSC-8779; D-Citronellol;(R)-(+)-beta-Citronellol; (+/-)-Citronellol;(+/-)-beta-Citronellol; DSSTox_CID_6726; DSSTox_RID_78201; DSSTox_GSID_26726; 68916-43-8; CAS-106-22-9; (+/-)-beta-Citronellol analytical standard; Citronellol (natural); (+-)-beta-citronellol; (+-)-CITRONELLOL; (R)-(+)-.beta.-Citronellol; UNII-565OK72VNF; (+/-)-beta-Citronellol, primary pharmaceutical reference standard; CCRIS 7452; Levo-citronellol; MFCD00063214; Citronellol Natural; EINECS 203-375-0; EINECS 247-737-6; MFCD00002935; BRN 1721507; ST069325; AI3-25080; beta-Citronellol, 95%; (+-)-beta;-Citronellol; EC 203-375-0; SCHEMBL21320; (S)-(-)-|A-Citronellol; 4-01-00-02188 (Beilstein Handbook Reference); 6-Octen-1-ol,7-dimethyl-; MLS002415719; 3,7-dimethyl-oct-6-en1-ol; CHEMBL395827; DTXSID3026726; CITRONELLOL, (+/-)-; HSDB 6805; .BETA.-CITRONELLOL [MI]; WLN: Q2Y1&3UY1&1; HMS2267B17; Citronellol, >=95%, FCC, FG; Tox21_202119; Tox21_300003; (+/-)-.BETA.-CITRONELLOL; BBL009826; BDBM50037035; s5584; STK085542; AKOS005393175; CCG-266265; CS-W010917; HY-W010201; (+/-)-3,7-dimethyl-6-octen-1-ol; .BETA.-CITRONELLOL, (+/-)-; NCGC00091348-01; NCGC00091348-02; NCGC00091348-03; NCGC00091348-04; NCGC00254145-01; NCGC00259668-01; AS-14688; BP-21491; SMR000112138; SY066737; ( inverted exclamation markA)-b-Citronellol; DB-060123; DB-074976; (+/-)-beta-Citronellol, analytical standard; FT-0604381; FT-0622896; FT-0623965; FT-0623966; FT-0693159; FT-0772868; 6-Octen-1-ol, 3,7-dimethyl-, (+/-)-; EN300-1270486; W-108771; W-109198; W-110227; Q27122080
|
|
| CAS | 106-22-9 | |
| PubChem CID | 8842 | |
| ChEMBL ID | CHEMBL395827 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 156.26 | ALogp: | 3.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 11 | QED Weighted: | 0.603 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.252 | MDCK Permeability: | 0.00001980 |
| Pgp-inhibitor: | 0.006 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.55 |
| 30% Bioavailability (F30%): | 0.101 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.948 | Plasma Protein Binding (PPB): | 93.48% |
| Volume Distribution (VD): | 2.272 | Fu: | 6.41% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.65 | CYP1A2-substrate: | 0.427 |
| CYP2C19-inhibitor: | 0.092 | CYP2C19-substrate: | 0.604 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.765 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.115 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.162 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.891 | Half-life (T1/2): | 0.593 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.018 | Human Hepatotoxicity (H-HT): | 0.573 |
| Drug-inuced Liver Injury (DILI): | 0.028 | AMES Toxicity: | 0.004 |
| Rat Oral Acute Toxicity: | 0.01 | Maximum Recommended Daily Dose: | 0.021 |
| Skin Sensitization: | 0.857 | Carcinogencity: | 0.224 |
| Eye Corrosion: | 0.915 | Eye Irritation: | 0.985 |
| Respiratory Toxicity: | 0.045 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
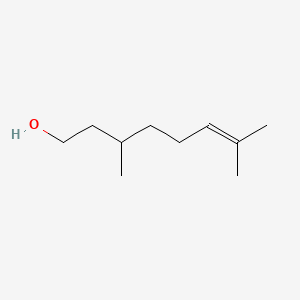
| ENC000229 | 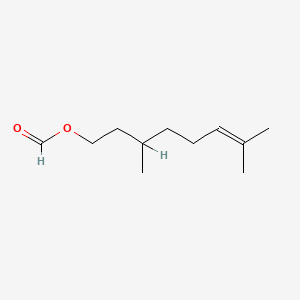 |
0.600 | D0M1PQ | 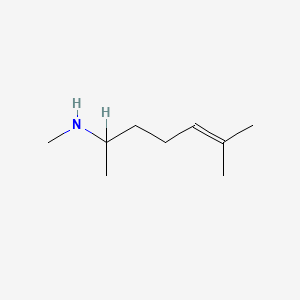 |
0.486 | ||
| ENC000319 | 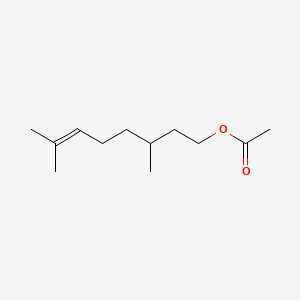 |
0.571 | D05XQE | 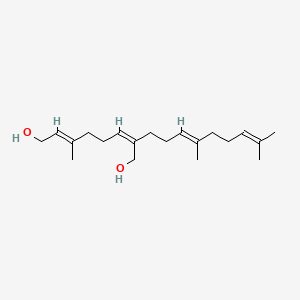 |
0.254 | ||
| ENC000230 | 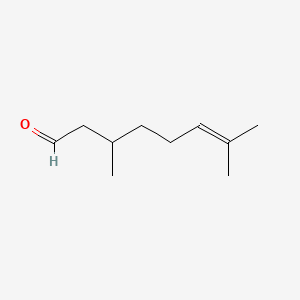 |
0.568 | D0Y3KG | 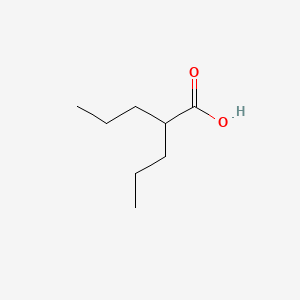 |
0.250 | ||
| ENC003366 | 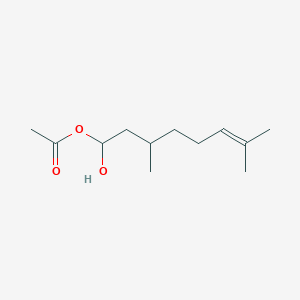 |
0.478 | D0C1QZ | 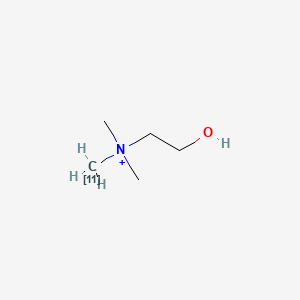 |
0.211 | ||
| ENC000396 | 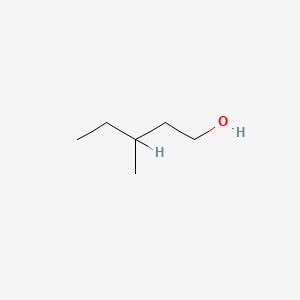 |
0.424 | D09XWD | 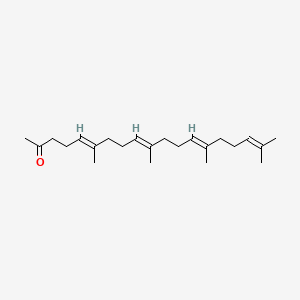 |
0.205 | ||
| ENC000846 | 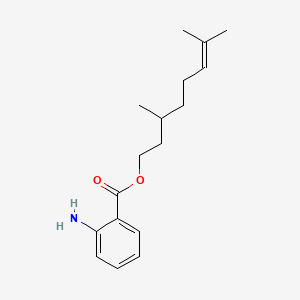 |
0.400 | D0ZK8H |  |
0.195 | ||
| ENC001150 |  |
0.391 | D03VFL |  |
0.189 | ||
| ENC000804 |  |
0.358 | D00WUF | 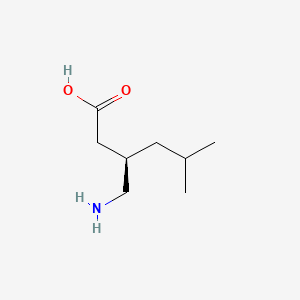 |
0.188 | ||
| ENC002844 | 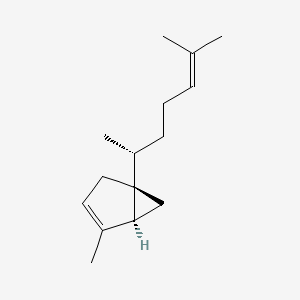 |
0.346 | D03CHT | 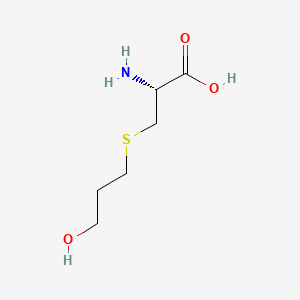 |
0.184 | ||
| ENC000796 | 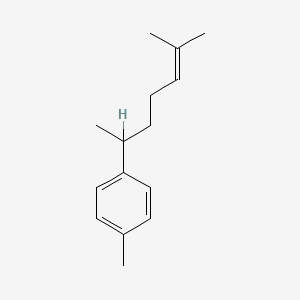 |
0.346 | D00FSV | 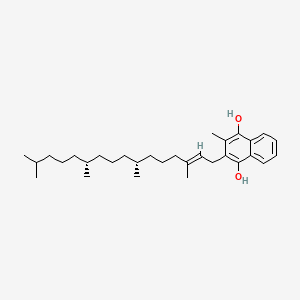 |
0.176 | ||