NPs Basic Information
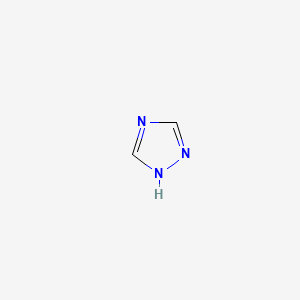
|
Name |
1,2,4-Triazole
|
| Molecular Formula | C2H3N3 | |
| IUPAC Name* |
1H-1,2,4-triazole
|
|
| SMILES |
C1=NC=NN1
|
|
| InChI |
InChI=1S/C2H3N3/c1-3-2-5-4-1/h1-2H,(H,3,4,5)
|
|
| InChIKey |
NSPMIYGKQJPBQR-UHFFFAOYSA-N
|
|
| Synonyms |
1,2,4-TRIAZOLE; 1H-1,2,4-Triazole; 288-88-0; 4H-1,2,4-triazole; Pyrrodiazole; s-Triazole; 1,2,4-1H-Triazole; 63598-71-0; MFCD00005228; NSC 83128; DTXSID6027131; 1H-[1,2,4]triazole; 10MS0Y1RDI; CHEBI:46077; NSC-83128; (1,2,4)-triazole; [1,2,4]-triazole; 736917-78-5; 1,2,4 triazole; 1H-1,2,4-triazol; 1,2,4-triazol; 4H-1,2,4-Triazole (VAN); Peptone, bacteriological; EINECS 206-022-9; UNII-10MS0Y1RDI; AI3-51031; HSDB 7860; 1,4-Triazole; 1,2,4triazole; 1,3,4-triazole; 1H-1,4-Triazole; 4H-1,4-Triazole; [1,2,4]triazole; 1,2, 4-triazole; 1,2,4,-triazole; [1,2,4]triazol; [1.2.4]-triazole; 1, 2, 4 triazole; [1,2,4]-triazol; 1,2,4-1h triazole; DSSTox_CID_7131; EC 206-022-9; 4H-[1,2,4]Triazole; DSSTox_RID_78318; DSSTox_GSID_27131; 1H-[1,2,4]-triazole; CHEMBL15571; 1,2,4-Triazole, 98%; NIOSH/XZ3807000; CHEBI:35550; EFINACONAZOLE METABOLITE H1; AMY40414; BCP20885; CS-D1150; NSC83128; TRIAZOLE, 1H,1,2,4-; ZINC5943507; Tox21_300113; CGA-71019; MFCD01941334; STK366100; 1,2,4-Triazole, analytical standard; 1H-1,2,4-TRIAZOLE [MI]; AKOS000120326; AKOS000269054; 1H-1,2,4-TRIAZOLE [HSDB]; DB03594; PS-9377; NCGC00247903-01; NCGC00254087-01; BP-12667; CAS-288-88-0; SY001414; DB-002017; BB 0267986; CS-0368547; FT-0607865; T0340; XZ38070000; EN300-20608; 88T880; E76126; T-6200; 1,2,4-Triazole, Vetec(TM) reagent grade, 98%; A819652; Q161300; F1918-0085; Z104479156
|
|
| CAS | 288-88-0 | |
| PubChem CID | 9257 | |
| ChEMBL ID | CHEMBL15571 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 69.07 | ALogp: | -0.6 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 41.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 5 | QED Weighted: | 0.473 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.512 | MDCK Permeability: | 0.00000942 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.142 |
| Human Intestinal Absorption (HIA): | 0.014 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.059 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.354 | Plasma Protein Binding (PPB): | 5.28% |
| Volume Distribution (VD): | 0.944 | Fu: | 91.45% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.194 | CYP1A2-substrate: | 0.177 |
| CYP2C19-inhibitor: | 0.124 | CYP2C19-substrate: | 0.097 |
| CYP2C9-inhibitor: | 0.014 | CYP2C9-substrate: | 0.524 |
| CYP2D6-inhibitor: | 0.363 | CYP2D6-substrate: | 0.029 |
| CYP3A4-inhibitor: | 0.294 | CYP3A4-substrate: | 0.155 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.138 | Half-life (T1/2): | 0.873 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.053 | Human Hepatotoxicity (H-HT): | 0.18 |
| Drug-inuced Liver Injury (DILI): | 0.968 | AMES Toxicity: | 0.054 |
| Rat Oral Acute Toxicity: | 0.347 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.93 | Carcinogencity: | 0.102 |
| Eye Corrosion: | 0.412 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.242 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000059 | 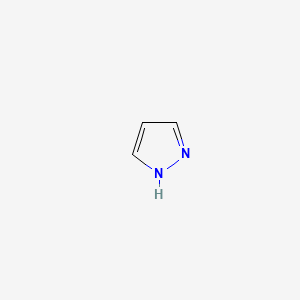 |
0.250 | D02NJA | 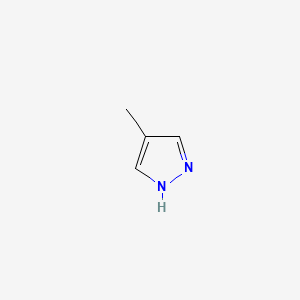 |
0.231 | ||
| ENC000011 | 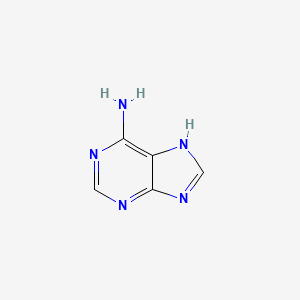 |
0.189 | D08IBS | 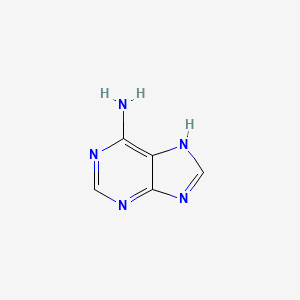 |
0.189 | ||
| ENC000721 | 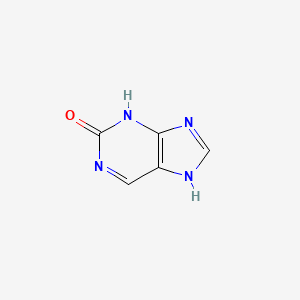 |
0.158 | D04KYY | 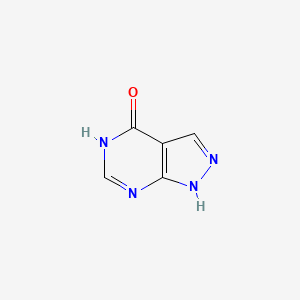 |
0.189 | ||
| ENC000138 | 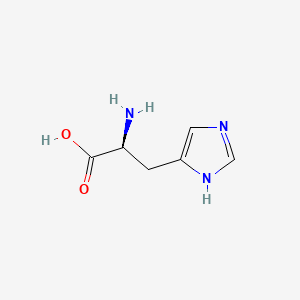 |
0.154 | D04USC | 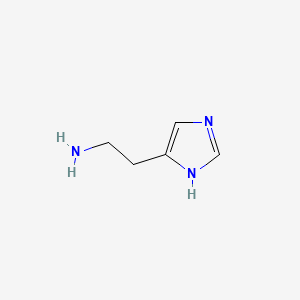 |
0.188 | ||
| ENC002160 | 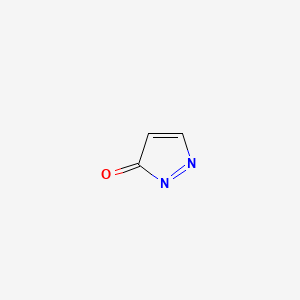 |
0.143 | D09UZO |  |
0.158 | ||
| ENC002316 | 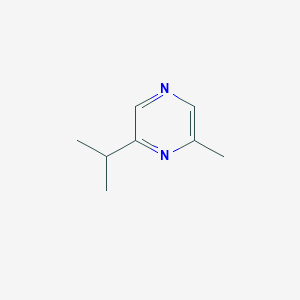 |
0.135 | D01OUE | 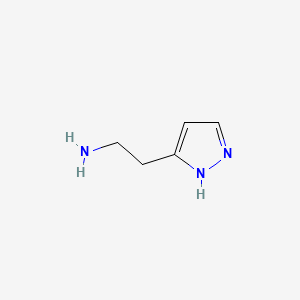 |
0.152 | ||
| ENC001061 |  |
0.121 | D0XF8W |  |
0.143 | ||
| ENC000352 |  |
0.121 | D08YIN |  |
0.129 | ||
| ENC000599 |  |
0.121 | D03OIW |  |
0.125 | ||
| ENC001065 |  |
0.113 | D02ZXM |  |
0.122 | ||