NPs Basic Information
|
Name |
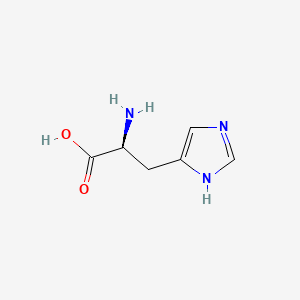
Histidine
|
| Molecular Formula | C6H9N3O2 | |
| IUPAC Name* |
(2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid
|
|
| SMILES |
C1=C(NC=N1)C[C@@H](C(=O)O)N
|
|
| InChI |
InChI=1S/C6H9N3O2/c7-5(6(10)11)1-4-2-8-3-9-4/h2-3,5H,1,7H2,(H,8,9)(H,10,11)/t5-/m0/s1
|
|
| InChIKey |
HNDVDQJCIGZPNO-YFKPBYRVSA-N
|
|
| Synonyms |
L-histidine; histidine; 71-00-1; H-His-OH; glyoxaline-5-alanine; L-(-)-Histidine; Anti-rheuma; Istidina; S-Histidine; (L)-Histidine; Histidine (VAN); HISTIDINE, L-; histidina; (S)-4-(2-Amino-2-carboxyethyl)imidazole; (2S)-2-amino-3-(1H-imidazol-4-yl)propanoic acid; L-Histidin; (S)-alpha-amino-1H-imidazole-4-propanoic acid; FEMA No. 3694; Histidine [USAN:INN]; Histidinum [INN-Latin]; (S)-Histidine; Histidina [INN-Spanish]; L-beta-(4-Imidazolyl)alanin; 4-(2-Amino-2-carboxyethyl)imidazole; his; L-hystidine; 1H-Imidazole-4-alanine, (S)-; L-Hisidine; (S)-2-Amino-3-(4-imidazolyl)propionsaeure; L-beta-(4-Imidazolyl)-alpha-alanin; (S)-alpha-Amino-1H-imidazole-4-propionic acid; L-His; L-Alanine, 3-(1H-imidazol-4-yl)-; AI3-26558; alpha-Amino-4(or 5)-imidazolepropionic acid; Histidine (L-Histidine); alpha-Amino-1H-imidazole-4-propionic acid, (S)-; 1H-Imidazole-4-propanoic acid, alpha-amino-, (S)-; (S)-a-Amino-1H-imidazole-4-propanoic acid; (S)-2-Amino-3-(4-imidazolyl)propionic acid; CHEBI:15971; 4QD397987E; NSC-137773; 7006-35-1; Histidinum; MFCD00064315; (2S)-2-amino-3-(imidazol-4-yl)propanoic acid; Histidine, monohydrochloride; HSDB 1810; EINECS 200-745-3; NSC 137773; UNII-4QD397987E; 1hsl; 1lag; [3H]histidine; Histidine,(S); [3H]-histidine; (2S)-2-amino-3-(1H-imidazol-4-yl)propanoic acid hydrochloride; Histidine (USP/INN); HISTIDINE [INN]; HISTIDINE [II]; HISTIDINE [MI]; L-Histidine (JP17); L-2-Amino-3-(4-imidazolyl)propionic acid; HISTIDINE [HSDB]; HISTIDINE [INCI]; HISTIDINE [USAN]; HISTIDINE [VANDF]; Lopac-H-8125; bmse000039; bmse000976; bmse001015; HISTIDINE [MART.]; L-HISTIDINE [FCC]; L-HISTIDINE [JAN]; L-Histidine (H-His-OH); amino-4-imidazoleproprionate; HISTIDINE [WHO-DD]; L-HISTIDINE [FHFI]; SCHEMBL3259; Lopac0_000566; US9138393, Histidine; US9144538, Histidine; (S)1H-Imidazole-4-alanine; CHEMBL17962; L-HISTIDINE [USP-RS]; (2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid; (S)-1H-Imidazole-4-alanine; BDBM7953; GTPL3310; GTPL4670; L-Histidine, non-animal source; Imidazole C-4(5) deriv. 5; amino-4-imidazoleproprionic acid; DTXSID9023126; HISTIDINE [EP MONOGRAPH]; amino-1H-imidazole-4-propanoate; HISTIDINE [USP MONOGRAPH]; L-Histidine, p.a., 98.5%; BDBM181118; L-Histidine, Cell Culture Reagent; 3-(1H-imidazol-4-yl)-L-Alanine; HY-N0832; ZINC6661227; amino-1H-imidazole-4-propanoic acid; AKOS015854051; AKOS026676613; AM81801; CCG-204656; CS-7781; DB00117; SDCCGSBI-0050549.P002; SERINE IMPURITY C [EP IMPURITY]; (S)-a-Amino-1H-imidazole-4-propanoate; NCGC00015518-01; NCGC00162189-01; NCGC00162189-02; NCGC00162189-05; AC-35086; AS-14171; (S)-alpha-Amino-1H-imidazole-4-propanoate; (S)-alpha-Amino-1H-imidazole-4-propionate; L-Histidine, BioUltra, >=99.5% (NT); H0149; L-Histidine, SAJ special grade, >=98.5%; S3989; EN300-57334; C00135; D00032; D70843; H-2310; L-Histidine, ReagentPlus(R), >=99% (TLC); M02982; L-Histidine, Vetec(TM) reagent grade, >=99%; (S)-2-amino-3-(1H-imidazol-4-yl)propanoic acid; 064H315; Q485277; B81AEDB0-EACA-4296-9BAB-52D60F137FFB; 1H-Imidazole-4-propanoic acid, .alpha.-amino-, (S)-; F8881-8926; F8889-0575; L-Histidine, certified reference material, TraceCERT(R); Z359369984; Histidine, European Pharmacopoeia (EP) Reference Standard; L-Histidine, United States Pharmacopeia (USP) Reference Standard; L-Histidine, Pharmaceutical Secondary Standard; Certified Reference Material; L-Histidine, cell culture tested, meets EP, USP testing specifications, from non-animal source
|
|
| CAS | 71-00-1 | |
| PubChem CID | 6274 | |
| ChEMBL ID | CHEMBL17962 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 155.15 | ALogp: | -3.2 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 92.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.564 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.56 | MDCK Permeability: | 0.00002080 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.067 |
| Human Intestinal Absorption (HIA): | 0.014 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.86 | Plasma Protein Binding (PPB): | 8.26% |
| Volume Distribution (VD): | 0.486 | Fu: | 96.71% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.012 | CYP1A2-substrate: | 0.123 |
| CYP2C19-inhibitor: | 0.046 | CYP2C19-substrate: | 0.059 |
| CYP2C9-inhibitor: | 0.013 | CYP2C9-substrate: | 0.59 |
| CYP2D6-inhibitor: | 0.018 | CYP2D6-substrate: | 0.054 |
| CYP3A4-inhibitor: | 0.057 | CYP3A4-substrate: | 0.044 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.116 | Half-life (T1/2): | 0.886 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.07 | Human Hepatotoxicity (H-HT): | 0.068 |
| Drug-inuced Liver Injury (DILI): | 0.027 | AMES Toxicity: | 0.017 |
| Rat Oral Acute Toxicity: | 0.164 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.815 | Carcinogencity: | 0.018 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.086 |
| Respiratory Toxicity: | 0.098 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001065 | 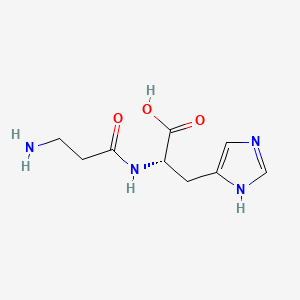 |
0.510 | D04USC | 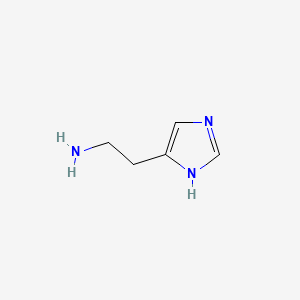 |
0.432 | ||
| ENC000918 | 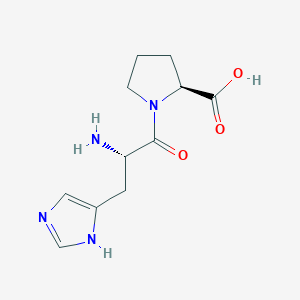 |
0.509 | D09NYU | 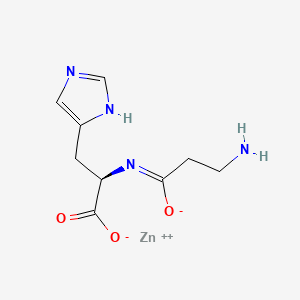 |
0.415 | ||
| ENC001902 | 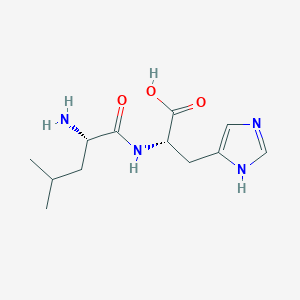 |
0.500 | D0R1CR |  |
0.370 | ||
| ENC000130 | 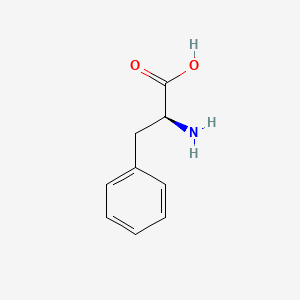 |
0.370 | D05EJG | 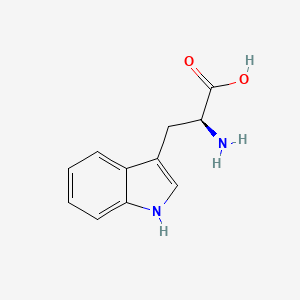 |
0.358 | ||
| ENC000140 | 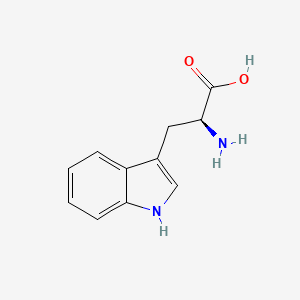 |
0.358 | D01CRB | 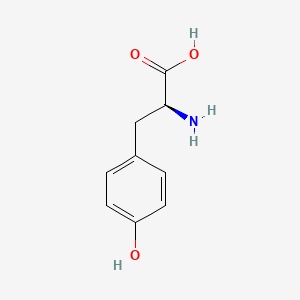 |
0.354 | ||
| ENC000129 | 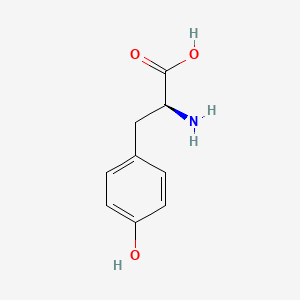 |
0.354 | D02UDJ | 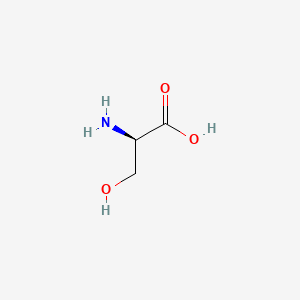 |
0.343 | ||
| ENC000127 | 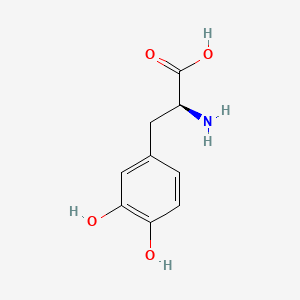 |
0.340 | D08HVR | 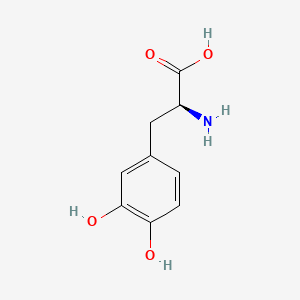 |
0.340 | ||
| ENC000325 | 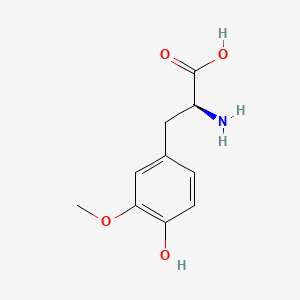 |
0.321 | D0P0QK | 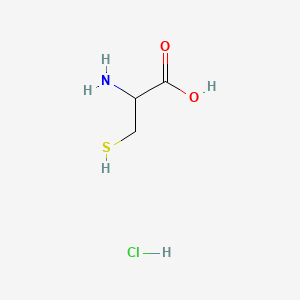 |
0.333 | ||
| ENC000137 | 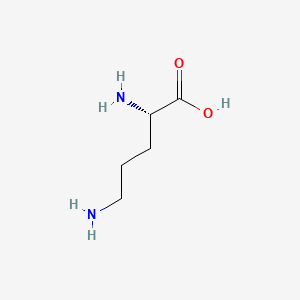 |
0.293 | D0EN0G | 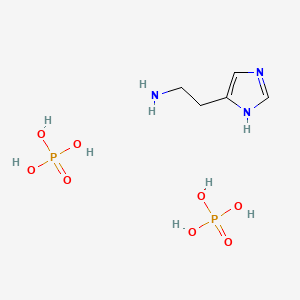 |
0.327 | ||
| ENC000550 | 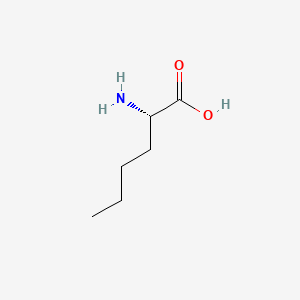 |
0.293 | D01OPV | 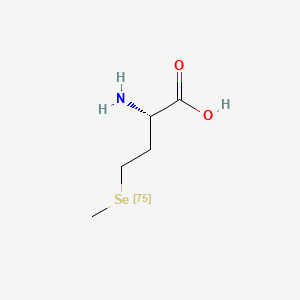 |
0.293 | ||