NPs Basic Information
|
Name |
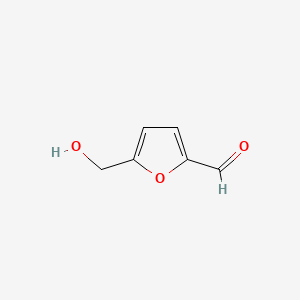
5-Hydroxymethylfurfural
|
| Molecular Formula | C6H6O3 | |
| IUPAC Name* |
5-(hydroxymethyl)furan-2-carbaldehyde
|
|
| SMILES |
C1=C(OC(=C1)C=O)CO
|
|
| InChI |
InChI=1S/C6H6O3/c7-3-5-1-2-6(4-8)9-5/h1-3,8H,4H2
|
|
| InChIKey |
NOEGNKMFWQHSLB-UHFFFAOYSA-N
|
|
| Synonyms |
5-HYDROXYMETHYLFURFURAL; 67-47-0; 5-Hydroxymethyl-2-furaldehyde; 5-(hydroxymethyl)furan-2-carbaldehyde; 5-(Hydroxymethyl)furfural; 5-(Hydroxymethyl)-2-furaldehyde; Hydroxymethylfurfural; 5-(Hydroxymethyl)-2-furfural; 5-Oxymethylfurfurole; 5-Hydroxymethylfuraldehyde; Hydroxymethylfurfurole; 5-(Hydroxymethyl)-2-furancarbonal; 2-Hydroxymethyl-5-furfural; 5-Hydroxymethylfuran-2-aldehyde; Hydroxymethylfurfuraldehyde; 5-Hydroxymethyl-2-formylfuran; Hydroxymethylfurfuralaldehyde; 5-Hydroxymethyl-2-furfural; 2-Furancarboxaldehyde, 5-(hydroxymethyl)-; 5-(Hydroxymethyl)-2-furfuraldehyde; 5-Hydroxymethylfurfuraldehyde; 5-(Hydroxymethyl)-2-furancarboxaldehyde; 5-(Hyddroxymethyl)furfurole; HMF; NSC-40738; 5-Hydroxymethyl-2-furancarboxaldehyde; 5-HYDROXYMETHYL-FURFURAL; Aes-103; 5-HMF; 2-Furaldehyde, 5-(hydroxymethyl)-; 5-Hydroxymethyl-2-furfuraldehyde; CHEBI:412516; 5-(Hydroxymethyl)furan-2-aldehyde; 5-Hydroxymethyl-2-furancarbaldehyde; MFCD00003234; NSC 40738; 5-(Hydroxymethyl)furfurole; 2-formyl-5-hydroxymethylfuran; 70ETD81LF0; 5-(Hydroxymethyl)-2-formylfuran; 5-Hydroxymethyl-furan-2-carbaldehyde; 5-hydroxymethyl furfural; DSSTox_CID_10428; DSSTox_RID_78848; DSSTox_GSID_30428; CAS-67-47-0; CCRIS 3160; EINECS 200-654-9; Aes 103; BRN 0110889; UNII-70ETD81LF0; HSDB 7982; 5-methylolfurfural; 5-(Hydroxymethyl)furan-2-carboxaldehyde; 5-hydromethyl-furfural; 5-Hydrxoymethylfurfural; 5-formylfurfuryl alcohol; 5HMF; 5-hydroxy methyl furfural; Epitope ID:136033; 5-Hydroxymethyl furaldehyde; CBiol_000485; SCHEMBL19176; 5-18-01-00130 (Beilstein Handbook Reference); MLS002454379; CHEMBL185885; QSPL 022; DTXSID3030428; GTPL10939; HYDROXYMETHYLFURFURAL, 5-; ZINC900788; 5-hydroxymethylfuran-2-carbaldehyde; BCP31207; CS-D1116; HY-Y0051; NSC40738; Tox21_201892; Tox21_303551; BBL100102; BDBM50487911; GEO-01528; ICCB3_000133; s3772; STL451297; 5-Hydroxymethyl-2-furaldehyde, 99%; 5-(hydroxymethyl)-2-furfural (HMF); AKOS005166879; AC-1262; CCG-266098; DB12298; GS-3074; 5-(hydroxymethyl)-furan-2-carbaldehyde; NCGC00091513-01; NCGC00091513-02; NCGC00257266-01; NCGC00259441-01; 5-(hydroxymethyl)-2-furan-carboxaldehyde; 5-(Hydroxymethyl)furfural, >=99%, FG; SMR000393981; SY010869; 5-(hydroxy methyl) 2-furan-carboxaldehyde; DB-007058; A9037; AM20100616; FT-0600483; H0269; 5-(HYDROXYMETHYL)-2-FURALDEHYDE [MI]; EN300-82764; 5-(Hydroxymethyl)furfural, analytical standard; 67H470; Q414606; Q-200545; 5-hydroxymethylfurfural; 5-(hydroxymethyl)-2-furaldehyde; F2191-0155; Z1238542812; 5-(hydroxymethyl)furan-2-carbaldehyde;5-(Hydroxymethyl)furfural
|
|
| CAS | 67-47-0 | |
| PubChem CID | 237332 | |
| ChEMBL ID | CHEMBL185885 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.11 | ALogp: | -0.6 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 50.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.601 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.677 | MDCK Permeability: | 0.00003530 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.214 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.444 |
| 30% Bioavailability (F30%): | 0.738 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.787 | Plasma Protein Binding (PPB): | 50.36% |
| Volume Distribution (VD): | 0.876 | Fu: | 75.18% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.473 | CYP1A2-substrate: | 0.117 |
| CYP2C19-inhibitor: | 0.052 | CYP2C19-substrate: | 0.101 |
| CYP2C9-inhibitor: | 0.011 | CYP2C9-substrate: | 0.487 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.684 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.231 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.185 | Half-life (T1/2): | 0.876 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.141 |
| Drug-inuced Liver Injury (DILI): | 0.177 | AMES Toxicity: | 0.866 |
| Rat Oral Acute Toxicity: | 0.063 | Maximum Recommended Daily Dose: | 0.011 |
| Skin Sensitization: | 0.274 | Carcinogencity: | 0.922 |
| Eye Corrosion: | 0.198 | Eye Irritation: | 0.986 |
| Respiratory Toxicity: | 0.319 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004043 | 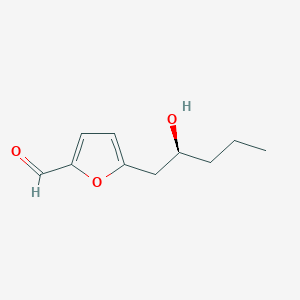 |
0.525 | D0E9CD |  |
0.250 | ||
| ENC000412 | 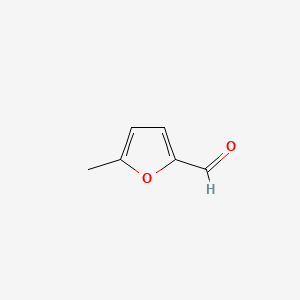 |
0.516 | D0IE1E | 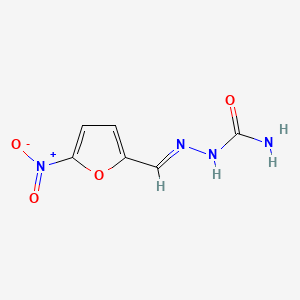 |
0.235 | ||
| ENC000748 | 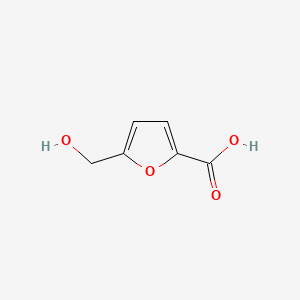 |
0.486 | D05OIS |  |
0.231 | ||
| ENC004044 | 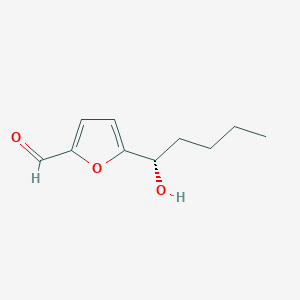 |
0.419 | D03CUF | 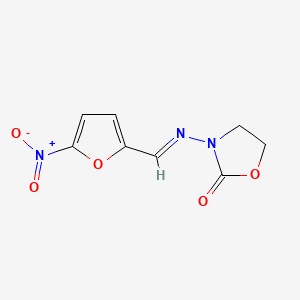 |
0.207 | ||
| ENC002238 | 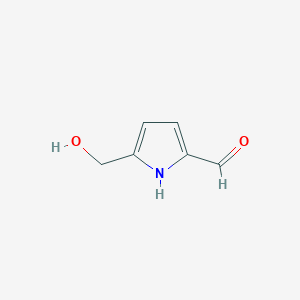 |
0.389 | D0R0BX | 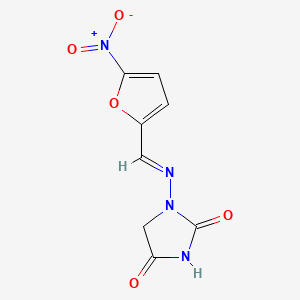 |
0.200 | ||
| ENC000189 |  |
0.324 | D08ZEB | 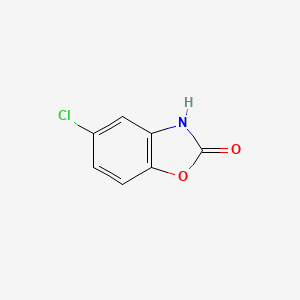 |
0.191 | ||
| ENC000190 | 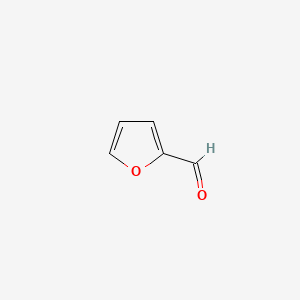 |
0.324 | D0B8WN | 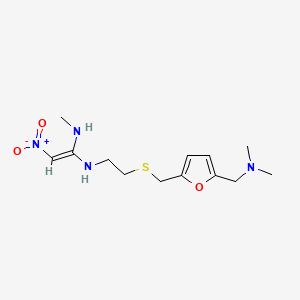 |
0.186 | ||
| ENC002334 | 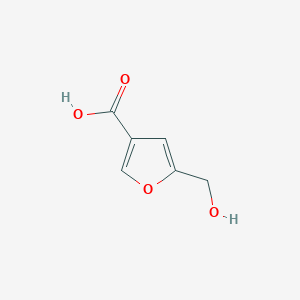 |
0.300 | D0FC1J | 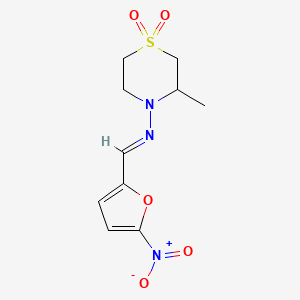 |
0.185 | ||
| ENC000101 | 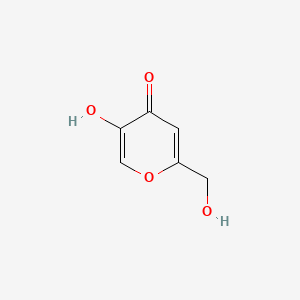 |
0.300 | D07HBX |  |
0.182 | ||
| ENC000166 | 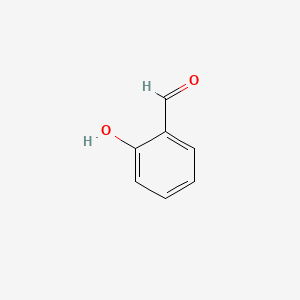 |
0.282 | D0C6OQ | 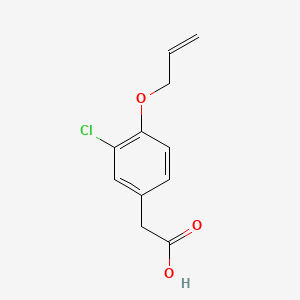 |
0.179 | ||