NPs Basic Information
|
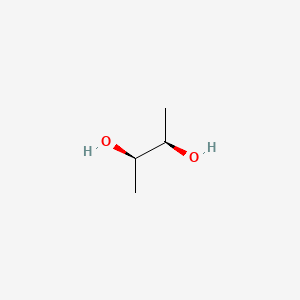
Name |
(R,R)-2,3-butanediol
|
| Molecular Formula | C4H10O2 | |
| IUPAC Name* |
(2R,3R)-butane-2,3-diol
|
|
| SMILES |
C[C@H]([C@@H](C)O)O
|
|
| InChI |
InChI=1S/C4H10O2/c1-3(5)4(2)6/h3-6H,1-2H3/t3-,4-/m1/s1
|
|
| InChIKey |
OWBTYPJTUOEWEK-QWWZWVQMSA-N
|
|
| Synonyms |
24347-58-8; (2R,3R)-butane-2,3-diol; (R,R)-2,3-butanediol; (2R,3R)-(-)-2,3-Butanediol; (R,R)-Butane-2,3-diol; 2,3-Butanediol, (-)-; (2R,3R)-2,3-butanediol; 6982-25-8; (R,R)-(-)-2,3-Butanediol; D-(-)-2,3-Butanediol; (R,R)-(-)-Butane-2,3-diol; Levo-2,3-Butanediol; (R,R)-2,3-Butylene glycol; 2,3-Butanediol, threo-; (R,R)-(-)-2,3-Butylene Glycol; (-)-2,3-butanediol; DL-2,3-Butanediol; (-)-(2R,3R)-Butanediol; 6510BGK6C5; OR02B2286A; 2,3-Butanediol, [R-(R*,R*)]-; 2,3-Butanediol #; BU3; 2,3-BUTANEDIOL, (R-(R*,R*))-; 2,3-Butanediol, (R*,R*)-(+-)-; UNII-6510BGK6C5; UNII-OR02B2286A; MFCD00064267; NSC-249246; (2R,3R)-rel-2,3-Butanediol; EINECS 246-186-9; (2r,3r)-butanediol; (r,r)-2,3 butanediol; D(-)-2,3-butanediol; D-2,3-BUTANEDIOL; L-(-)-2,3-Butanediol; THREO-2,3-BUTANEDIOL; (-)-(r,r)-2,3-butanediol; CHEBI:16982; rel-(2R,3R)-2,3-Butanediol; 2,3-Butanediol, (R*,R*)-; DTXSID801026532; DTXSID801031371; ZINC901616; NSC15829; (+/-)-2,3-BUTANEDIOL; (2R,3R)-(-)-2,3-butandiol; (R,R)-(-)-2,3-Dihydroxybutane; (2R, 3R)(-)-2,3-butanediol; BBL101946; NSC-15829; s3333; STL555743; (2R, 3R)-(-)-2,3-butanediol; AKOS015907648; AKOS016015450; CS-W016670; HY-W015954; 2,3-BUTANEDIOL, (+/-)-; 2,3-BUTANEDIOL, (2R,3R)-; AC-26496; AS-57289; BP-30189; 2,3-BUTANEDIOL, (2R,3R)-REL-; 2,3-BUTYLENE GLYCOL DL-THREO-FORM; (-)-(2R,3R)-2,3-BUTANEDIOL; DB-009316; (2R,3R)-(-)-2,3-Butanediol, 97%; B1161; 2,3-BUTANEDIOL, (2R,3R)-(-)-; 2,3-BUTYLENE GLYCOL D(-)-THREO-FORM; C03044; C91323; EN300-141851; 2,3-BUTANEDIOL, (R*,R*)-(+/-)-; 2,3-BUTYLENE GLYCOL DL-THREO-FORM [MI]; 347B588; A817243; 2,3-BUTYLENE GLYCOL D(-)-THREO-FORM [MI]; J-500969; J-506903; (2R,3R)-butane-2,3-diol;(2R,3R)-2,3-Butanediol; Q27102161
|
|
| CAS | 24347-58-8 | |
| PubChem CID | 225936 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 90.12 | ALogp: | -0.9 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 0 |
| Heavy Atoms: | 6 | QED Weighted: | 0.482 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.707 | MDCK Permeability: | 0.00069846 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.14 |
| Human Intestinal Absorption (HIA): | 0.007 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.007 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.238 | Plasma Protein Binding (PPB): | 13.50% |
| Volume Distribution (VD): | 0.919 | Fu: | 81.93% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.074 | CYP1A2-substrate: | 0.561 |
| CYP2C19-inhibitor: | 0.024 | CYP2C19-substrate: | 0.821 |
| CYP2C9-inhibitor: | 0.005 | CYP2C9-substrate: | 0.734 |
| CYP2D6-inhibitor: | 0.005 | CYP2D6-substrate: | 0.317 |
| CYP3A4-inhibitor: | 0.003 | CYP3A4-substrate: | 0.126 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.552 | Half-life (T1/2): | 0.813 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.021 | Human Hepatotoxicity (H-HT): | 0.026 |
| Drug-inuced Liver Injury (DILI): | 0.061 | AMES Toxicity: | 0.014 |
| Rat Oral Acute Toxicity: | 0.013 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.061 | Carcinogencity: | 0.026 |
| Eye Corrosion: | 0.193 | Eye Irritation: | 0.984 |
| Respiratory Toxicity: | 0.017 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000016 | 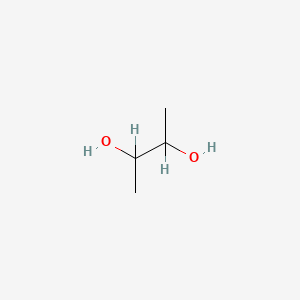 |
1.000 | D08QGD | 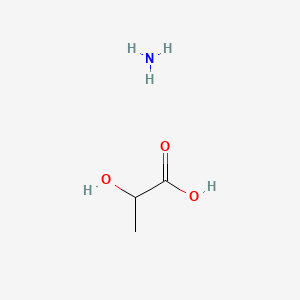 |
0.286 | ||
| ENC000874 | 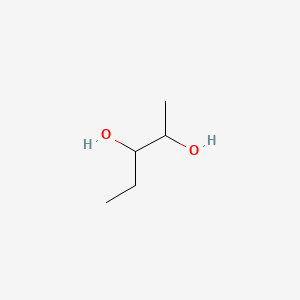 |
0.500 | D00ZOF | 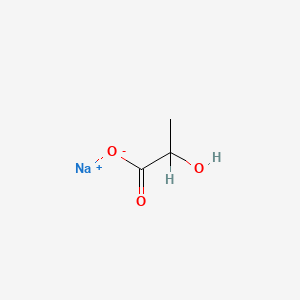 |
0.227 | ||
| ENC000824 | 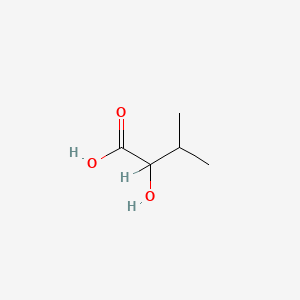 |
0.391 | D02OAV | 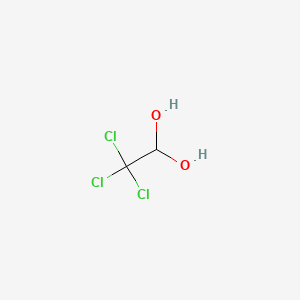 |
0.217 | ||
| ENC004318 | 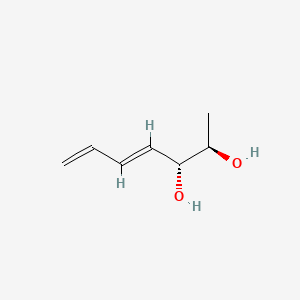 |
0.333 | D00WUF | 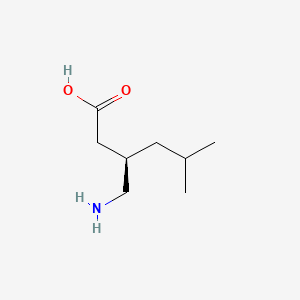 |
0.206 | ||
| ENC000147 | 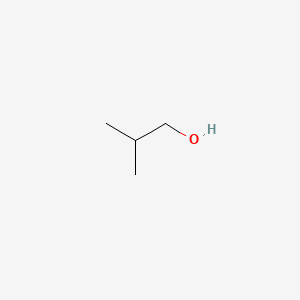 |
0.333 | D0I8FI | 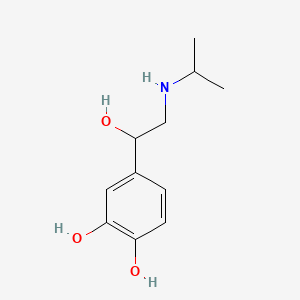 |
0.205 | ||
| ENC000057 | 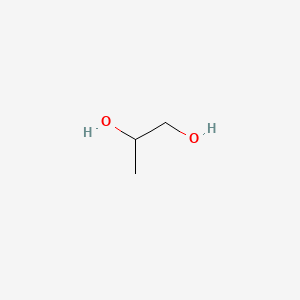 |
0.333 | D02UFG | 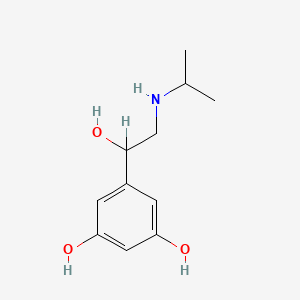 |
0.205 | ||
| ENC000814 | 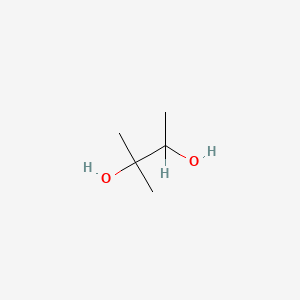 |
0.333 | D04EYC | 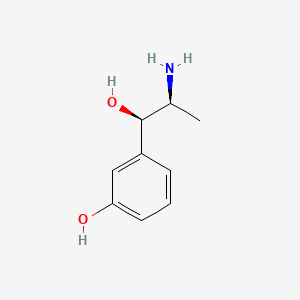 |
0.184 | ||
| ENC000037 | 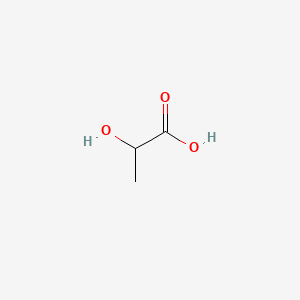 |
0.300 | D08HUC | 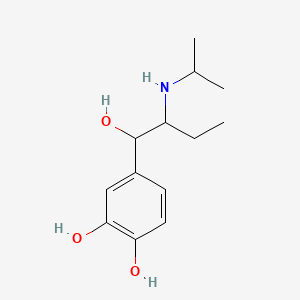 |
0.184 | ||
| ENC000149 | 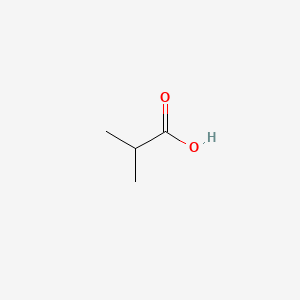 |
0.300 | D09PUL | 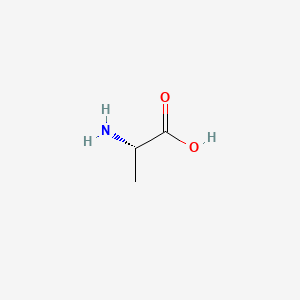 |
0.182 | ||
| ENC000010 | 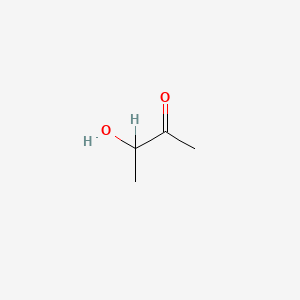 |
0.300 | D0Q9YT |  |
0.180 | ||