NPs Basic Information
|
Name |
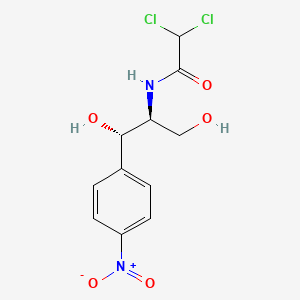
Dextramycin
|
| Molecular Formula | C11H12Cl2N2O5 | |
| IUPAC Name* |
2,2-dichloro-N-[(1S,2S)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide
|
|
| SMILES |
C1=CC(=CC=C1[C@@H]([C@H](CO)NC(=O)C(Cl)Cl)O)[N+](=O)[O-]
|
|
| InChI |
InChI=1S/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18)/t8-,9-/m0/s1
|
|
| InChIKey |
WIIZWVCIJKGZOK-IUCAKERBSA-N
|
|
| Synonyms |
Dextramycine; Dextramycin; L-(+)-Threo-chloramphenicol; 134-90-7; L-threo-Chloramphenicol; (+)-Chloramphenicol; L-Chloramphenicol; Dextromycetin; L-threo-Chloroamphenicol; 2,2-dichloro-N-[(1S,2S)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide; U0PWV2Z3IW; 137731-89-6; L-threo-(1S,2S)-1-p-Nitrophenyl-2-dichloroacetamido-1,3-propanediol; CHLORAMPHENICOL LEVO; Acetamide, 2,2-dichloro-N-((1S,2S)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-; threo-Chloramphenicol, l-; 2787-09-9; 2,2-dichloro-N-((1S,2S)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl)acetamide; Acetamide, 2,2-dichloro-N-((1S,2S)-2-hydroxy-1-(hydroxymethyl)-2-(4-ni trophenyl)ethyl)-; rel-2,2-Dichloro-N-((1S,2S)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl)acetamide; EINECS 205-161-2; UNII-U0PWV2Z3IW; CAS-56-75-7; starbld0016561; L-CHLOROAMPHENICOL; L-threo-N-Dichloracetyl-1-p-nitrophenyl-2-amino-1,3-propanediol; SCHEMBL49057; Acetamide, 2,2-dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)-, (S-(R*,R*))-; CHEMBL1437336; CHLORAMPHENICOL, L-THREO-; DTXSID40158453; HMS3867F03; ZINC113386; MFCD01733852; (1S,2S)-2-(2,2-Dichloroacetamido)-1-(4-nitrophenyl)-1,3-propanediol; (S-(R*,R*))-2,2-Dichloro-N-(2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl)acetamide; NCGC00016249-01; Acetamide, 2,2-dichloro-N-(beta-hydroxy-alpha-(hydroxymethyl)-p-nitrophenethyl)-, L-threo-(+)-; AS-13302; EN300-7411953; J-006623; Q27290527; N-[(alphaS,betaS)-alpha-(Hydroxymethyl)-beta-hydroxy-4-nitrophenethyl]dichloroacetamide; [R-(R*,R*)]-2,2-dichloro-N-[2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]ethanamide; ACETAMIDE,2,2-DICHLORO-N-((1S,2S)-2-HYDROXY-1-(HYDROXYMETHYL)-2-(4-NI TROPHENYL)ETHYL)-; LCL
|
|
| CAS | 134-90-7 | |
| PubChem CID | 92099 | |
| ChEMBL ID | CHEMBL1437336 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 323.13 | ALogp: | 1.1 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 115.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 20 | QED Weighted: | 0.415 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.616 | MDCK Permeability: | 0.00231626 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.172 |
| Human Intestinal Absorption (HIA): | 0.011 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.27 | Plasma Protein Binding (PPB): | 59.20% |
| Volume Distribution (VD): | 0.718 | Fu: | 40.90% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.028 | CYP1A2-substrate: | 0.134 |
| CYP2C19-inhibitor: | 0.035 | CYP2C19-substrate: | 0.844 |
| CYP2C9-inhibitor: | 0.005 | CYP2C9-substrate: | 0.435 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.221 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.208 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.684 | Half-life (T1/2): | 0.526 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.103 | Human Hepatotoxicity (H-HT): | 0.042 |
| Drug-inuced Liver Injury (DILI): | 0.054 | AMES Toxicity: | 0.355 |
| Rat Oral Acute Toxicity: | 0.04 | Maximum Recommended Daily Dose: | 0.011 |
| Skin Sensitization: | 0.48 | Carcinogencity: | 0.084 |
| Eye Corrosion: | 0.007 | Eye Irritation: | 0.304 |
| Respiratory Toxicity: | 0.907 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000122 | 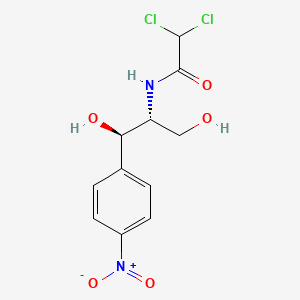 |
1.000 | D0X6IU | 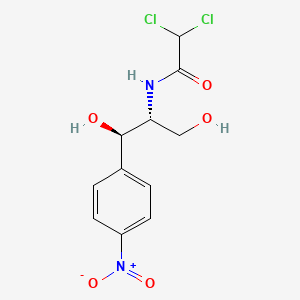 |
1.000 | ||
| ENC000034 |  |
0.361 | D03YLZ |  |
0.662 | ||
| ENC002666 |  |
0.317 | D05HFY |  |
0.316 | ||
| ENC001493 |  |
0.317 | D0J9ZR | 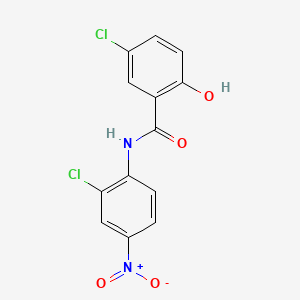 |
0.310 | ||
| ENC006123 | 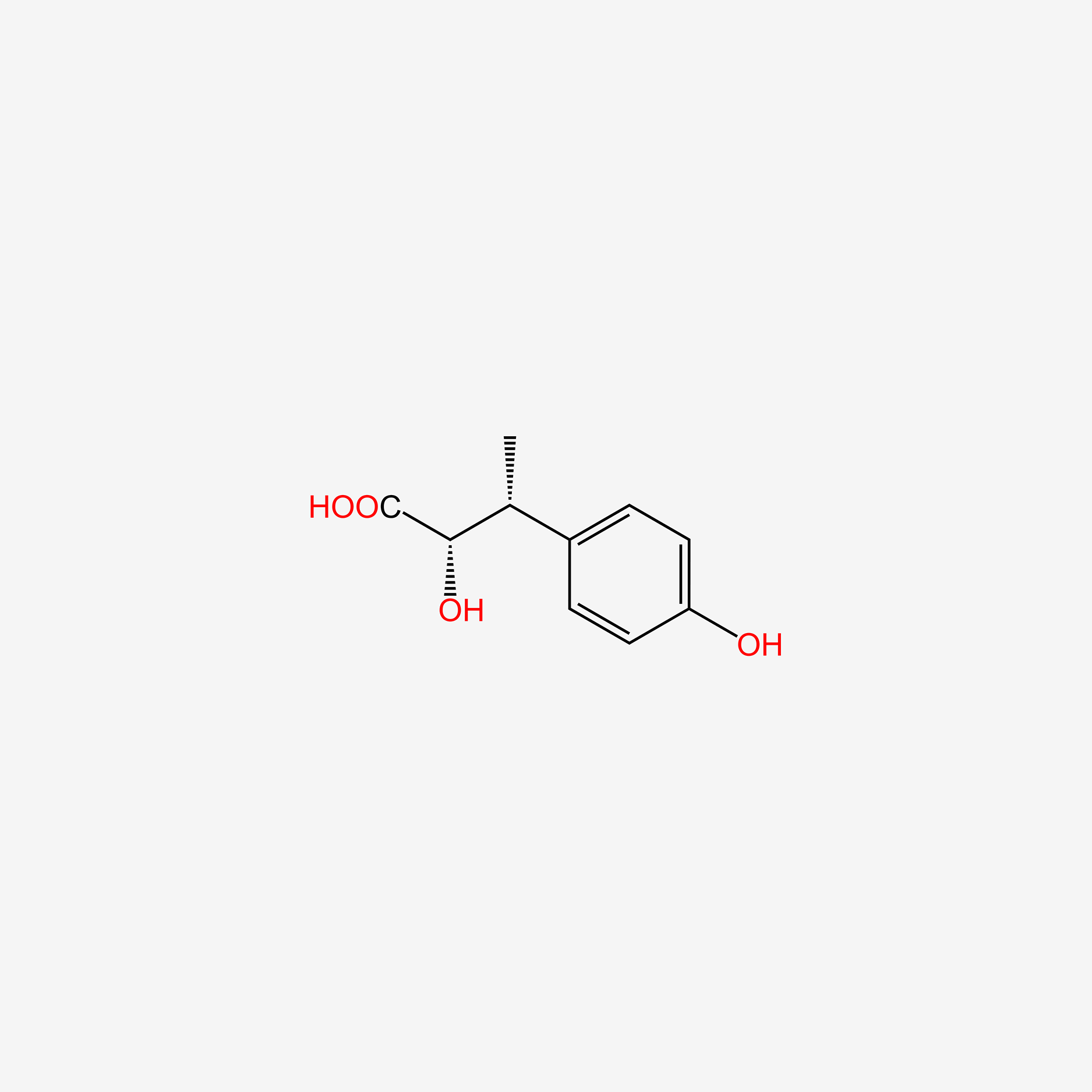 |
0.304 | D02WAB | 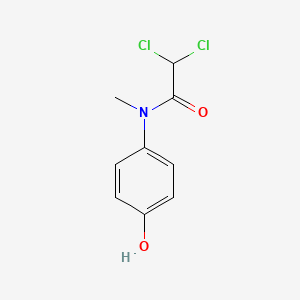 |
0.286 | ||
| ENC001364 | 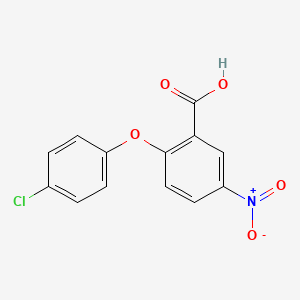 |
0.286 | D01AJY | 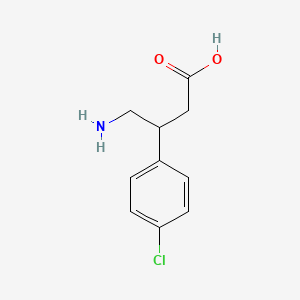 |
0.282 | ||
| ENC005262 | 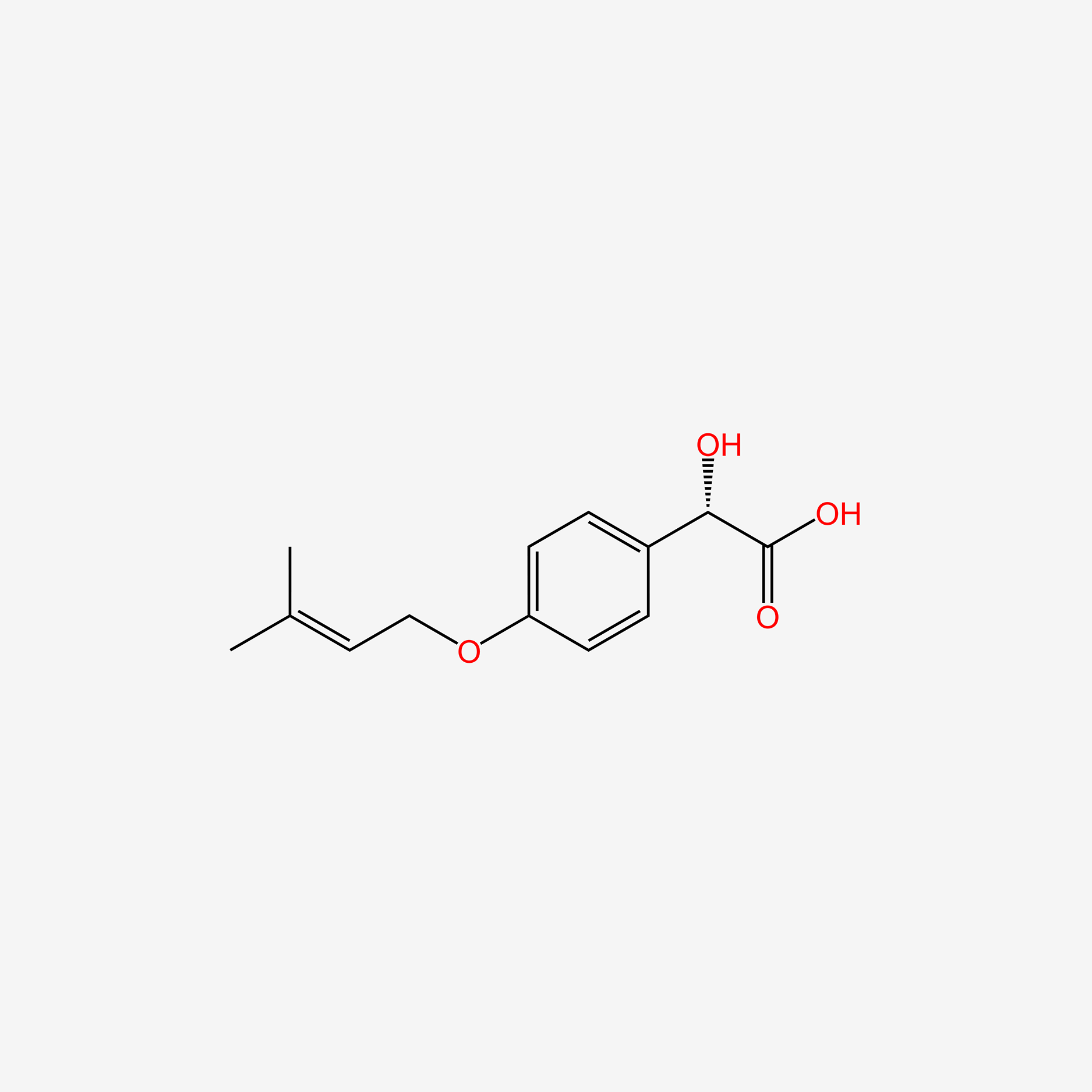 |
0.269 | D0R1QE | 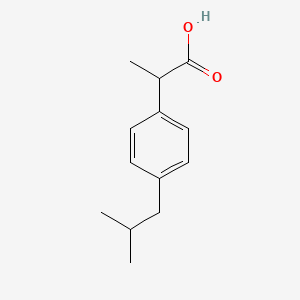 |
0.274 | ||
| ENC005325 | 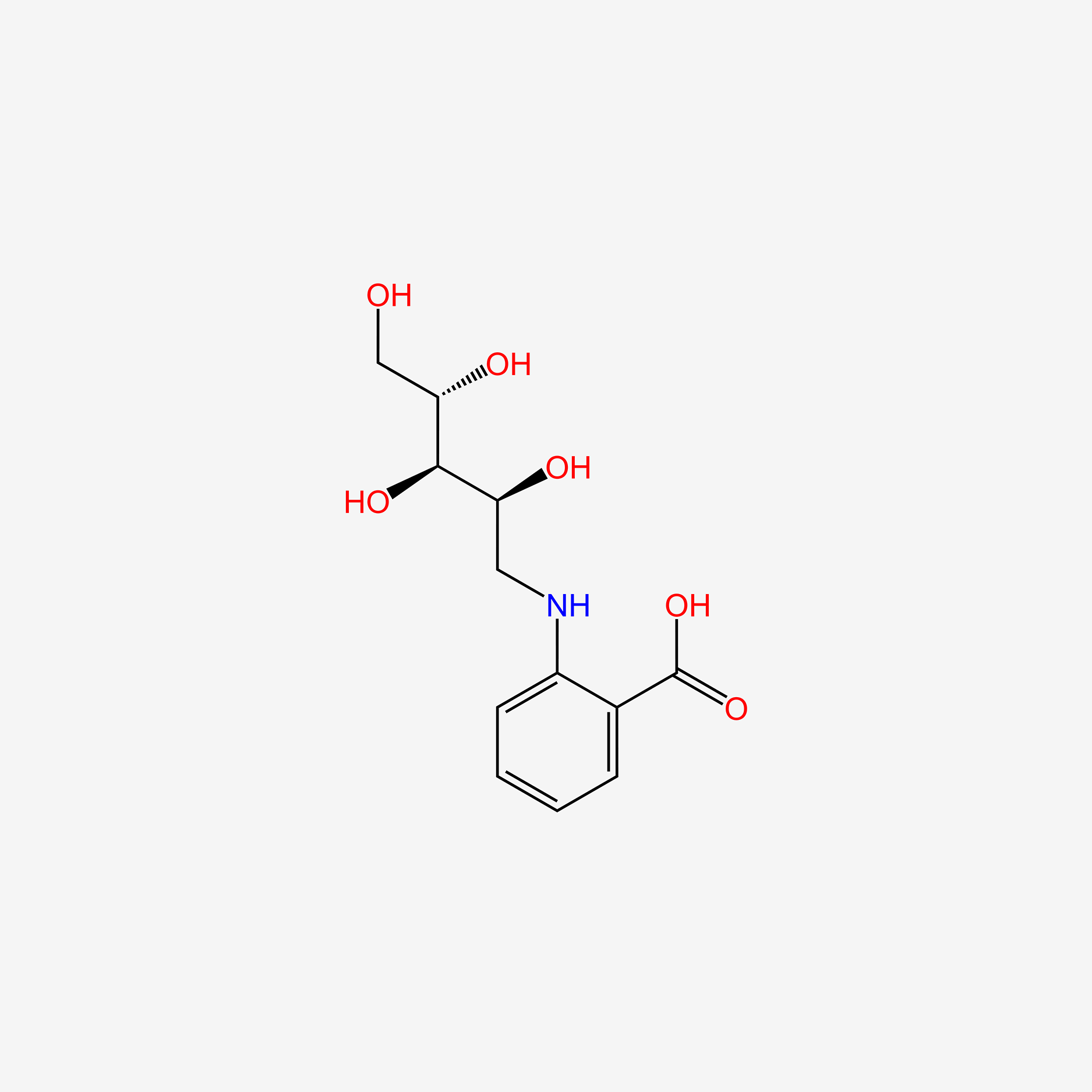 |
0.253 | D04VMT | 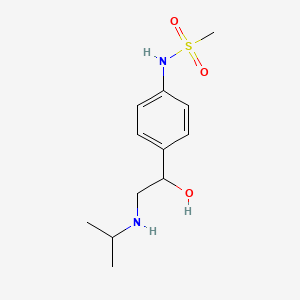 |
0.263 | ||
| ENC003949 |  |
0.244 | D08HUC | 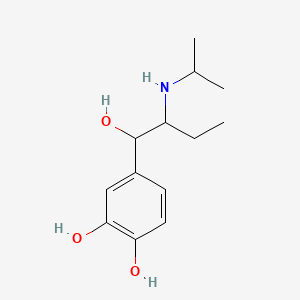 |
0.256 | ||
| ENC000129 | 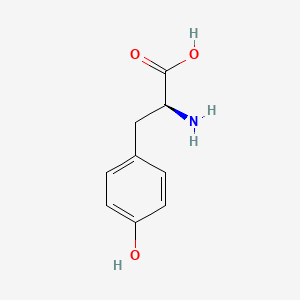 |
0.239 | D00LFB | 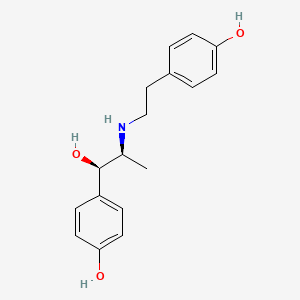 |
0.244 | ||